INTRODUCTION
Approximately 48–70% of patients with obesity exhibit obstructive sleep apnoea (OSA)1. The condition is characterised by partial or complete collapse of the upper airways, resulting in repeated episodes of snoring, hypoxia, and fragmented sleeping. Obstructive sleep apnoea interrupts the function of the sympathetic nervous system, thus influencing hemodynamic status, resulting in episodes of hypoxia followed by reoxygenation. In the long term, repeated alternation between the two states leads to chronic inflammation, oxidative stress, and various metabolic complications2.
In addition to dyslipidemia, insulin resistance, and glucose intolerance, OSA often increases the risk of developing non-alcoholic fatty liver disease (NAFLD)2, i.e. the excessive accumulation of fats or lipoproteins, particularly triglycerides, in the hepatic tissues3; this is particularly the case in obese patients2. It has been estimated that NAFLD is present in around 78% of severe OSA sufferers4. NAFLD can result in a number of hepatic manifestations ranging from simple steatosis to more aggressive non-alcoholic chronic steatohepatitis, the latter being a risk factor for the development of hepatic cirrhosis or carcinoma5.
The treatment of choice for OSA involves the use of continuous positive airway pressure (CPAP) to stabilize the airways during sleep; by doing so, the treatment reduces the risk of apnea or hypopnea. Regular use of CPAP in OSA patients has been found to result in reduced daytime sleepiness, improved quality of life, and enhanced daytime functioning6.
However, no approved pharmacological therapy for NAFLD currently exists7. Furthermore, while CPAP is known to improve sleeping problems, snoring and hypoxia related to OSA, the evidence regarding the effects of CPAP on liver enzyme levels in obese OSA patients remains unclear8.
It has been found that, in addition to lifestyle modifications, CPAP could be regarded as a gold standard treatment for OSA, and may ameliorate OSA-induced metabolic and cardiovascular dysfunctions7. However, another recent study indicated that CPAP therapy alone did not alter NAFLD-associated elevation of alanine transaminase (ALT) or aspartate transaminase (AST)9, and such lifestyle modifications in OSA patients with NAFLD require further trials6,9.
A simpler form of exercise which is not considered to be a lifestyle change is free walking. Free walking offers a number of benefits as a form of energy expenditure; for example, it does not require any equipment, such as a treadmill, and it can be applied in rural and remote locations where easy access to equipment is difficult. Free walking is usually performed to improve muscle coordination, poor balance, bone health, and metabolic conditions. Compared to limited space treadmill walking, free over-ground walking is more natural, safe, and widely appreciated by people with different conditions; as such, it is characterised by better adherence than other aerobic activities.
The use of CPAP alone appears to have little effect on weight loss, metabolic dysfunction and markers of OSA10. Therefore, the aim of this study is to determine the effect of supplementing CPAP with free walking with as a lifestyle modification on liver enzyme levels, fatigue severity, triglyceride (TG) levels, and sleeping quality in OSA patients with NAFLD.
MATERIALS AND METHODS
Design
Forty OSA patients with NAFLD were recruited to take part in this randomised study (clinical trials registration number NCT06508190). The participants, patients with OSA and NAFLD, were recruited from the Chest Diseases Outpatient Clinic and Internal Diseases Outpatient Clinic of the 6th October Hospital between 12 July and 1 December 2024.
Ethics
The procedures used in the trial (assessments, interventions, and outcomes of OSA participants with NAFLD) were approved by the local Research Ethics Approval Committee (Pharos University in Alexandria) with the reference 03202405263221. All participants gave their consent to participate, in accordance with the Helsinki agreement, among other ethical standards.
To be eligible for inclusion, the participants had to fulfil the following criteria: OSA patients with NAFLD diagnosed by ultrasonography11, no prior use of CPAP therapy while sleeping, no participation in a supervised or unsupervised weight loss program in the previous 24 weeks. The following exclusion criteria were applied: orthopedic issues/defects in lower limbs, liver transplantation, systemic illnesses (diabetes and hypertension), alcoholic fatty liver, intake of sleeping or anti-psychotic pills, respiratory/neurological dysfunctions, previous bariatric surgeries, cardiac/renal defects, liver metastasis, pregnancy, circadian desynchrony (such as shift workers), viral hepatitis, and autoimmune diseases.
Randomization
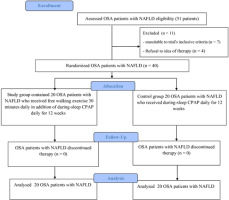
Forty OSA patients with NAFLD were recruited and then randomly assigned to a group receiving CPAP alone (n = 20) or another group receiving CPAP plus free walking (aerobic exercise group, n = 20). The allocation was randomised by the closed envelope technique (Figure 1). The researcher allocating participants to the groups did not participate in any evaluation or treatment procedure related to this study.
Interventions
CPAP therapy
The CPAP therapy was performed using an ESmart-GII Automatic CPAP (BMC CPAP, China). All participants in both groups were instructed to use the CPAP device with nasal mask for at least four hours every night for the 12-week programme. During this programme, the participants received daily calls from the researchers at nightfall to confirm that they must use the device while sleeping.
Free walking exercise
In the aerobic training group, all participants performed a continuous free walking exercise in addition to receiving CPAP. Each walking session was performed in the open air for half an hour, seven times a week, throughout the 12-week programme. The walking session itself took place outside daily working hours, if the patient was employed by the government or private firm. While walking, the heart rate was monitored using a PULSOX-2020 heart rate monitor (China), with the rate maintained below sixty percent of the target heart rate.
The authors asked any close relatives living with the patients to check during the night to confirm the use of the CPAP device. Daily walking sessions were supervised by the authors through the Imo app: a video calling program downloaded onto the participant’s mobile phone.
Measurements
The serum levels of hepatic enzymes, aspartate transaminase (AST) and alanine transaminase (ALT; the primary outcome), serum triglyceride (TG), and body mass index (BMI) were assessed. Fatigue was measured using the fatigue severity scale (FSS); this is a valid scale scored from 9 to 63 with a higher score indicating a higher perception of OSA-induced fatigue12.
The probability of sleeping or dozing off during eight common sedentary tasks was evaluated using the Epworth sleepiness scale (ESS). Each task has four response options: 0 (would never doze/sleep) to 3 (high likelihood of dozing/sleeping). In all OSA patients with NAFLD, the maximal score of ESS was 24, with a low ESS scores indicating a low risk of sleepiness13.
The quality of sleep was determined using the Pittsburgh Sleep Quality Index (PSQI): a reliable and subjective instrument for assessing sleeping quality in patients with sleep disorders. The maximum score is 21, with a low score indicating a high chance of good sleep quality14-16.
The details of CPAP therapy or free walking exercise were not disclosed by the authors to the outcome examiners: the outcome assessors were blinded to the allocation of participants to groups.
Sample size calculation
At 80% power, using G*Power German analysis, the effect size of the ALT measurement, the primary study outcome, was estimated to be 1.04. This sample size analysis was based on 16 pilot-test OSA patients with NAFLD. Hence, the minimum recommended group size was 16 patients. To prevent a 25% decline, the authors added four OSA patients with NAFLD to each group.
Statistical analysis
The normality of the data (basic data and outcomes) was confirmed by the Smirnov test. At the start of the intervention, the unpaired T-test was used to compare age and waist circumference between groups, while the Chi-square test was used to compare sex ratios. ANOVA was used to compare outcomes of ALT, TG, FSS, AST, PSQI, BMI, and ESS within or between groups. A p-value < 0.05 was assumed to indicate significance. All analyses were performed using SPSS 18.
RESULTS
The included group ranged from 41 to 64 years of age. The degree of OSA ranged from moderate to severe: i.e. an apnea-hypopnea index between 15 and 45 events/hour as determined by sleep laboratory analysis10. The body mass index (BMI) was below 35 kg/m2. No significant differences in age, BMI, waist size/circumference or sex distribution were found between the two groups at the start of the study (Table 1).
Table 1
Participant characteristics, i.e. OSA patients with NAFLD (mean ± SD)
| Group characteristics | CPAP plus free walking group | CPAP group | p-value |
|---|---|---|---|
| Age (years) | 48.45 ± 5.84 | 50.60 ± 6.21 | 0.266 |
| BMI (Kg/m2) | 31.84 ± 1.57 | 32.13 ± 1.62 | 0.564 |
| Waist circumference (cm) | 113.45 ± 6.73 | 112.95 ± 7.43 | 0.824 |
| Sex ratio | 16♂: 4♀ | 14♂ : 6♀ | 0.465* |
In addition, at the beginning of the intervention, no significant intergroup differences were observed regarding the primary outcome (ALT) or the secondary outcomes (FSS, AST, PSQI, TG, and ESS) (Table 2).
Table 2
Outcome values at the end of the programme
| CPAP plus free walking group (mean ± SD) | CPAP group (mean ± SD) | Intergroup significance (p-value) | ||
|---|---|---|---|---|
| BMI (Kg/m2) | Initial value, i.e. at baseline | 31.84 ± 1.57 | 32.13 ± 1.62 | 0.564 |
| Final value, i.e. after 12 weeks | 28.8 ± 2.12 | 31.91 ± 2.02 | < 0.001* | |
| Intragroup significance (p-value) | < 0.001* | 0.386 | ||
| Alanine transaminase (IU/L) | Initial (at baseline) | 36.27 ± 4.04 | 38.16 ± 6.18 | 0.259 |
| Final (after 12 weeks) | 25.18 ± 3.48 | 34.11 ± 5.91 | < 0.001* | |
| Intragroup significance (p-value) | < 0.001* | < 0.001* | ||
| Aspartate transaminase (IU/L) | Initial (at baseline) | 27.71 ± 3.49 | 28.15 ± 3.46 | 0.694 |
| Final (after 12 weeks) | 18.31 ± 3.03 | 24.46 ± 3.79 | < 0.001* | |
| Intragroup significance (p-value) | < 0.001* | < 0.001* | ||
| Triglycerides (mg/dL) | Initial (at baseline) | 164.15 ± 24.52 | 173.50 ± 20.60 | 0.200 |
| Final (after 12 weeks) | 141.10 ± 24.95 | 168.10 ± 19.11 | < 0.001* | |
| Intragroup significance (p-value) | < 0.001* | 0.025 | ||
| Pittsburgh Sleep Quality Index | Initial (at baseline) | 10.15 ± 1.75 | 11.50 ± 2.91 | 0.084 |
| Final (after 12 weeks) | 3.05 ± 0.99 | 6.90 ± 1.68 | < 0.001* | |
| Intragroup significance (p-value) | < 0.001* | < 0.001* | ||
| Epworth sleepiness scale | Initial (at baseline) | 10.10 ± 1.83 | 10.50 ± 1.70 | 0.479 |
| Final (after 12 weeks) | 5.65 ± 1.38 | 7.45 ± 1.79 | 0.001* | |
| Intragroup significance (p-value) | < 0.001* | < 0.001* | ||
| Fatigue severity scale | Initial (at baseline) | 44.65 ± 7.07 | 46.15 ± 8.28 | 0.542 |
| Final (after 12 weeks) | 30.45 ± 6.24 | 37.50 ± 6.79 | 0.002* | |
| Intragroup significance (p-value) | < 0.001* | < 0.001* |
The CPAP-only group demonstrated a significant improvement in the primary outcome (ALT), and the secondary outcomes (FSS, AST, PSQI, TG, and ESS); however, no significant change in BMI was observed. The aerobic training group, i.e. CPAP plus walking, showed significant improvements in ALT, TG, FSS, AST, PSQI and ESS, as well as BMI; this group also achieved more favourable ALT, FSS, AST, TG, PSQI and ESS at the end of the study than the CPAP-alone group (Table 2). At the end of the intervention, the CPAP and free walking group demonstrated significantly better ALT, TG, FSS, AST, PSQI, BMI, and ESS compared to the CPAP-alone group (Table 2).
DISCUSSION
Our findings indicate that free walking exercise further supplemented the effects of CPAP on ALT, TG, FSS, AST, PSQI, BMI, and ESS in OSA patients with NAFLD. This is not surprising, as regular aerobic exercise, such as free walking, has been suggested to improve sleep quality in OSA patients. Many theories have been proposed to account for this.
The first theory is that exercise may improve sleep quality by reducing BMI. Such a reduction is associated a lower risk of upper airway collapsibility, and hence fragmented sleep patterns17,18. The second concerns the ability of exercise to reduce fluid accumulation in the lower limbs; this reduction is not only strongly associated with greater improvements in sleeping quality19,20 but also with low complaints of repeated physical fatigue while performing daily activities. Another theory is that exercise can increase respiratory muscle recruitment in OSA patients, which in turn enhances the activation of smooth muscles in the upper airways; this activation lowers the resistance of the upper airways during sleep19. Finally, another theory concerns the role of exercise in increasing body temperature, which is believed to facilitate the onset of sleep by the hypothalamus, which controls various mechanisms of the sleep process21.
In addition, physical exercise not only improves reported sleep problems and low quality of sleep, but also corrects OSA-induced metabolic dysfunctions18. Exercise induces a reduction of total and regional body fats22 which are one of the main risk factors for the development of OSA-associated metabolic dysfunctions. One of the regional groups of body fats known to be reduced by exercise is the visceral adipose tissue (VAT). Such reduction has repeatedly been found to lower the levels of OSA-associated pro-inflammatory markers23, which in turn reduces peripheral insulin resistance (PIR). This exercise-induced reduction in PIR lowers the excessive delivery of fats and glucose to hepatic tissues, elevates the rates of fatty acid oxidation, decreases the synthesis of new free fatty acids, inhibits the formation of new TG, and reduces hepatocellular and mitochondrial damage24; together, these factors result in an improvement in NAFLD in OSA patients.
Lifestyle changes are known to lower elevated leptin levels. This effect is has been attributed to both lowering the elevated hepatic triglyceride content25 and decreasing the levels of NAFLD induced by OSD; it is also believed to correct hyperleptinemia-induced impairment in neuroanatomic interactions, which are necessary for ensuring stable breathing during sleep26.
Exercise has been found to have local effects on hepatic tissues. More specifically, it has been demonstrated to slow down hepatic stellate cell proliferation, enhance immune system activation to produce local anti-inflammatory biomarkers, and limit the spread of inflammation to new hepatocytes. These effects not only account for the reported improvements in ALT and AST level in OSA patients, but also improves liver function to prevent the conversion of NAFLD to live cirrhosis27.
Our present results achieved for CPAP as sole therapy are in line with those of a previous study indicating that six-month CPAP therapy significantly lowered ALT and AST in OSA patients with NAFLD, but without any improvement in triglyceride (TG) level28. It has also been noted that two to three-year adherence to CPAP therapy yielded a significant improvement in mean ALT and AST in patients with OSA-induced fatty liver; however, unlike our findings, TG did not improve29.
The present study investigated the use of free walking training as a supplement to CPAP for improving sleep quality, liver enzyme levels, and fatigue perception in OSA patients with NAFLD. These findings are confirmed by a number of previous studies. Research on OSA patients found a 24-week weight-loss program incorporating increased physical activities to significantly reduce TG, ALT and BMI30, while another 16-month programme of regular physical aerobic activities significantly improved liver enzyme levels (Gamma-glutamyl Transferase), TG, BMI, and ESS31. In addition, a three-month programme of non-surgical weight loss was found to alleviate sleep breathing disorders and their associated metabolic dysfunctions, including NAFLD32. Also, an eight-week intervention including urban walking as a lifestyle change in OSA patients significantly improved BMI, fatigue perception indicated by improved performance of the six-minute walking test, and ESS33, and an eight-week exercise rehabilitation significantly improved ESS, BMI, and physical fatigue score34. Another eight-week study on OSA patients based on combined aerobic and resistance training significantly improved TG and ESS35, while another 12-week aerobic exercise training programme improved ESS and PSQI36.
In addition, a 2022 randomized clinical trial examined the effect of lifestyle changes and CPAP in 89 overweight or obese men with a moderate-to-severe degree of OSA. The participants were categorized into two groups: one which received CPAP, and another which received CPAP and lifestyle changes (i.e. sportive and nutritional support). After eight weeks, it was reported that the combined intervention significantly improved ESS and PSQI compared with CPAP alone18. Elsewhere, a 20-week combination of aerobic exercise and CPAP treatment was found to significantly improve BMI, metabolic indices (lipoproteins, blood glucose, and insulin resistance), and physical capacity assessed via a six-minute walk test37. Furthermore, an eight-week programme based on CPAP combined with aerobic exercise was more effective in improving ESS in OSA patients than CPAP alone38.
Some other studies are in partial agreement with our current findings. Onemonth exercise training significantly improved physical fatigue, PSQI and ESS in OSA patients, but not TG, suggesting that longer programme might be needed to improve TG level39. Also, while a six-month study conducted in 2020 yielded improved BMI and ESS in OSA patients, no change was observed in TG, AST or ALT; because the lifestyle interventions were not supervised by the authors40. Also, another short intervention (eight weeks) based on CPAP or aerobic exercise alone did not improve PSQI in OSA patients41.
Limitations
The study did not track ALT, TG, FSS, AST, PSQI, BMI or ESS after ending the interventions. Such longer-term follow ups would be recommended for future studies on OSA patients with NAFLD.
In addition, the sample size was rather small, with only 20 patients per group, and the intervention was relatively short, i.e. 12 weeks. Furthermore, the study lacks objective adherence data regarding CPAP usage and inflammatory cytokine or biomarker levels, and no fatty liver imaging or biopsy was performed for NAFLD staging. Finally, some potential bias may be associated with the use of tele-supervised exercise.
CONCLUSIONS
Adding free walking exercise to CPAP therapy may improve ALT, TG, FSS, AST, PSQI, BMI, and ESS in OSA patients with NAFLD. This supports the integration of low-intensity aerobic activities and exercises into OSA management strategies for patients with comorbid NAFLD. Our findings indicate that free walking offers a number of benefits in inter alia hepatic, metabolic, and sleep-related health domains, which may be particularly relevant in OSA patients with NAFLD.