INTRODUCTION
Restless Leg Syndrome (RLS) is a neurological sensorimotor disorder characterized by an uncontrollable urge to move the limbs, particularly during periods of rest or inactivity, most often in the evening or at night. Patients typically describe the sensations as creeping, crawling, tingling, or pulling, predominantly affecting the lower limbs and often extending from the ankles to the knees1,2. These symptoms are strongly linked to sleep issues, such as trouble falling asleep and staying asleep, thus leading to low sleep quality, daytime tiredness and challenges in everyday functioning. RLS exhibits a daily rhythm, intensifying during the night and somewhat improving in the morning3-5. It is often linked with diseases like diabetes mellitus, chronic kidney disease, iron deficiency, Parkinson’s disease, multiple sclerosis, and pregnancy. Its risk factors include obesity, physical inactivity and smoking, and deficiencies in iron, folic acid and magnesium6.
The pathophysiology of RLS is complex, involving central dopaminergic dysfunction and altered iron metabolism in the basal ganglia; emerging evidence also suggests roles for neuroinflammation, neurodegeneration and neurotransmitter imbalance7-10. A notable comorbid condition is type 2 diabetes mellitus (T2DM), with 8.8% to 27% of diabetic patients also estimated to experience RLS11-15. This association is primarily attributed to diabetic peripheral neuropathy and chronic hyperglycemia, which may exacerbate RLS symptoms by impairing peripheral nerve function and altering central neurochemical pathways1.
There is growing evidence suggesting that various pharmacological and non-pharmacological approaches are effective for managing RLS. However, while pharmacological therapies can offer symptomatic relief, they are often accompanied by side effects and risk of augmentation16.. Therefore, there is increasing interest in non-pharmacological strategies for RLS management. Interventions such as physical activity, yoga, contrast hydrotherapy, and neuromodulation have shown promising results17-19.
Among these approaches, aerobic exercise has been widely studied and reported to reduce RLS symptom severity, improve sleep quality, and enhance physical performance and quality of life20,21. An analysis of 19 studies involving both humans and animals suggests that aerobic exercise may alleviate RLS through increased β-endorphin release and improved dopaminergic signaling22. Aerobic exercise has been demonstrated to alleviate RLS symptom severity20,21, and improve sleep quality, sleep duration and daytime sleepiness, as well as physical performance and quality of life23,24. However, such training has been associated with a greater risk of hypoglycemia, particularly in insulin-dependent patients, and late evening aerobic sessions might disrupt sleep onset due to elevated sympathetic activity25.
Similarly, resistance training programs have been found to achieve significant improvements in RLS symptoms and sleep quality through enhanced neuromuscular function, increased lower limb strength, and better regulation of dopaminergic pathways in the pathophysiology of RLS26,27. Such training supports central dopamine activity by stimulating the release of brain-derived neurotrophic factor (BDNF), and exerts anti-inflammatory effects by reducing the levels of circulating cytokines, such as IL-6 and TNF-α28. Improved endothelial function and peripheral circulation may further relieve sensory symptoms, while the better glycemic control associated with regular resistance exercise helps mitigate diabetic neuropathy, a key contributor to RLS in diabetes29. On the other hand, resistance training can potentially exacerbate the nocturnal symptoms of RLS by causing muscle soreness and transient discomfort. It may also be associated with poor adherence in unsupervised settings, increased joint stress in overweight individuals, and transient hypertensive responses in those with cardiovascular comorbidities.
Although aerobic and resistance (strength) training have independently demonstrated beneficial effects on RLS symptoms, existing studies have primarily focused on non-diabetic populations or examined the effects of each intervention in isolation. Moreover, no study has systematically compared the effectiveness of aerobic and strength training in patients with diabetes with RLS. This represents a critical knowledge gap, as diabetic individuals may respond differently to aerobic and resisted exercises due to altered metabolic, vascular, and neuromuscular profiles.
To date, no randomized clinical trial has directly compared the effects of aerobic and strength training on RLS symptoms, sleep quality, and daytime alertness in patients with T2DM under controlled and monitored conditions. To address this, the present randomized controlled trial compares the effects of aerobic and resistance training on RLS symptom severity, sleep quality, and daytime sleepiness in patients with T2DM and comorbid RLS. Importantly, the study takes into account potential limitations, such as the risk of hypoglycemia associated with aerobic exercise, and musculoskeletal strain or poor adherence with resistance training, and the participants were monitored carefully to maximize the benefits and minimize the risks in this population.
It was hypothesized that aerobic exercise and resistance training have significantly different effects on RLS severity, sleep quality, and daytime sleepiness in diabetic patients with RLS.
MATERIALS AND METHODS
Study design
The study was performed as a two-armed, parallel, randomized clinical trial. The research was conducted at the Center of Diabetes and Liver Disease and Fitness Lounge Gym, Islamabad from February 2020 to December 2020. Approval was given by the Research Ethical Committee of Riphah International University before performing the intervention (RIPHAH/RCRS/REC/Letter-00660). All ethical standards and the Helsinki Declaration of Ethical Principles for Medical Research were followed. The study was registered with ClinicalTrials.gov (NCT04316052). Written informed consent was obtained from every participant.
Participants
The study included male and female patients aged between 40 and 60 years; all had obtained a diagnosis of Type 2 Diabetes Mellitus (ICD-10: E11) at least five years previously, and were solely receiving oral medications. Eligible participants were required to exhibit lower limb muscle strength of at least 3+/5 on the Manual Muscle Testing (MMT) scale. Restless Legs Syndrome (RLS) was diagnosed according to the International Restless Legs Syndrome Study Group (IRLSSG) criteria (ICD-11 code 8A80). Confirmation required the presence of all core IRLSSG features including: (i) an urge to move the limbs, usually associated with uncomfortable sensations; (ii) motor restlessness; (iii) symptom exacerbation during periods of rest or inactivity; (iv) partial or complete relief with movement; and (v) symptoms that worsen in the evening or at night.
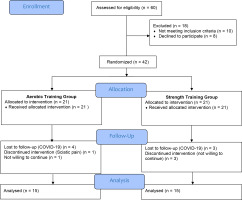
Participants were excluded if they had taken part in intensive exercise programs within the last year or were receiving insulin treatment; they were also excluded if they reported the following: diagnosed psychiatric conditions, known iron deficiency anemia, diagnosed sleep disorders other than RLS (e.g., obstructive sleep apnea), body mass index (BMI) > 35 kg/m², regular consumption of alcohol, excessive nicotine use. In addition, severe comorbid conditions such as heart failure, stroke, active infectious disease, cognitive impairment or communication difficulties, or lower limb musculoskeletal injuries (e.g., fractures) or major visual/hearing impairments were also cause for exclusion. The CONSORT diagram for the study is presented in Figure 1.
Outcome measure tools
The International Restless Leg Syndrome Rating Scale (IRLS) is an international tool for determining the severity of RLS. It aims to assess the frequency, severity and impact of RLS on daily life based on a 10-item questionnaire. Its primary outcome measure is the RLS total severity score, calculated by the total of all components. A lower score indicates mild to moderate symptoms and a high score more severe symptoms, with a maximum score of 40. Studies have reported a Cronbach’s alpha value ranging from 0.81 to 0.95, showing high reliability30,31.
The Pittsburgh Sleep Quality Index (PSQI) evaluates the quality of sleep during the previous month based on seven components of subjective sleep quality, sleep duration, sleep latency, habitual sleep efficiency, sleep disturbances, sleeping medication use, and dysfunction during the day. A number between 0 (no difficulty) and 3 (severe difficulty) is assigned to each component. The total PSQI score thus ranges from 0 to 21. A global score of greater than 5 shows poor sleep quality. Previous studies have found the PQSI to have a Cronbach’s alpha coefficient ranging from 0.70 to 0.83 and a good test-retest correlation coefficient for global score ranging from 0.77 to 0.85, showing a stable measure32.
The Epworth Sleepiness Scale (ESS) measures daytime sleepiness. It is an eight-question self-administered questionnaire. It has a four-point response scale indicating the typical likelihood of dozing off or falling asleep across eight activities. Each item is scored from 0 (would never doze) to 3 (high chance of dozing), for a total score range of 0 to 24. A higher score shows severe excessive daytime sleepiness. The ESS has shown strong test–retest reliability (r = 0.83)33,34.
Sample size
The study sample size was calculated with Open Epi, using the mean International RLS Study Group (IRLSSG) scores 10.7 ± 7.6 for the exercise group and 18.3 ± 7.8 for the controls, with a confidence interval (α) of 95% and 80% power, based on Giannaki et al.23. To obtain a significant difference, the exact sample size calculated using Open Epi was 34. Therefore, to account for potential attrition, 42 participants were recruited in the study.
Randomization and allocation procedure
The study was performed as a single-blinded randomized controlled trial, in which the outcome assessor was blinded to group allocation to minimize assessment bias. The principal investigator first screened potential participants based on predefined inclusion and exclusion criteria: the investigator is an experienced physiotherapist with over 12 years of clinical and research experience.
Sample selection was performed by non-probability convenience sampling. Participants were randomly assigned to either Group A (Aerobic Training, n = 21) or Group B (Resistance Training, n = 21) with a 1:1 allocation ratio; this allocation was facilitated by the Online Research Randomizer tool (https://www.randomizer.org/) which assigned each participant randomly into either group. The assessments were performed at baseline and after programme completion (i.e. after six weeks) by a trained therapist who was blinded to the group assignments. The researcher performing the intervention was not blinded to group allocation, as they were responsible for administering the prescribed treatment protocols. The data was coded and analyzed using SPSS Version 21.
Exercise interventions
In accordance with the exercise prescription guidelines established by the American College of Sports Medicine (ACSM), individualized exercise programs were designed for both intervention groups. Participants in Group A underwent aerobic training, while those in Group B received resistance training. Each intervention session lasted 45 minutes and was conducted three times per week, totaling 24 sessions over a six-week period.
Each session comprised a structured warm-up (eight minutes), the main exercise component (30 minutes), and a cool-down period (seven minutes). The warm-up included four minutes of stationary marching in a standing position, two minutes of ankle pumps, and two minutes of slow-paced walking.
The cool-down protocol involved two minutes of slow walking at a self-selected comfortable pace, two minutes of light marching in place (performed either seated or standing), followed by three minutes of static stretching targeting the lower limbs. Stretches included calf, hamstring, and quadriceps muscles, each held for 15-30 seconds, to facilitate gradual recovery, reduce heart rate, and prevent musculoskeletal stiffness. The main training component varied by groups. Outcome assessments were conducted at baseline and after the completion of the six-week intervention.
Aerobic training protocol
The participants in Group A engaged in a structured aerobic exercise program comprising two components: a 15-minute walk followed by 15 minutes of stationary cycling. The cycling was performed on either an upright or recumbent ergometer, with exercise intensity maintained between 50% and 80% of the age-predicted maximum heart rate (HRmax) in accordance with established aerobic training guidelines. The cycling first starting with low resistance for the first three minutes, maintaining moderate resistance for next four to twelve minutes, and gradually decreasing resistance in the last three minutes to prepare for cool-down.
Strength training protocol
The participants in this group performed a structured lower limb strengthening program consisting of the following machine-based exercises: horizontal leg press, leg extension, leg curl, hip adduction, hip abduction, seated rotary calf press. Each exercise was performed in two sets of eight to twelve repetitions, with a 2 s hold during both the concentric (lifting) and eccentric (lowering) phases. The initial load was set at 50% of the estimated one-repetition maximum (1-RM). Exercise progression was implemented based on individual performance. When participants were able to complete two sets of 10–12 repetitions with maximal exertion for two consecutive sessions, the resistance was gradually increased. Progression was guided by the Borg Rating of Perceived Exertion (RPE) scale, aiming for an exertion level between 10 and 1323.
Statistical Analysis
Statistical analysis was performed through SPSS 21. The Shapiro-Wilk test was applied to assess the normality of the data. The IRLS, PSQI, and ESS data was found to be normally distributed (p > 0.05); hence, the independent sample t-test was used for the between-group analysis, and the paired t-test for the within-group analysis. For the RLS, PSQI, and ESS sub-components, the data was non-normally distributed; therefore, the Mann-Whitney U-test and Wilcoxson sign rank test were applied for sub-component analysis. Homogeneity of variance for parametric tests was confirmed using Levene’s test. Baseline characteristics were compared to ensure group homogeneity prior to intervention, thereby controlling for confounding variable.
RESULTS
A total of 60 participants were assessed for eligibility; of these, 18 participants were excluded because they did not meet the inclusion criteria. Thus, 42 participants were recruited at baseline. Out of these 42 participants, 30 participants completed the intervention protocol. Adherence to the intervention protocol was measured by session attendance. To maintain adherence, regular reminders were given. Participants were considered adherent if they attended at least 85% of the prescribed sessions. Throughout the intervention period, no serious adverse events were reported in either group. During the six-week exercise program, 12 participants dropped out during the treatment: one participant developed sciatica and chose not to continue the intervention, seven dropped out due to COVID-19-related restrictions, and four were not willing to continue the intervention. As a result, 30 participants completed the exercise and post-intervention assessment. Consequently, the calculated sample size of 34 was not achieved and data from 30 participants were analyzed.
In total, the final group included 19 female (59.1%) and 11 male (40.9%) participants. The overall mean age of individuals was 54.09 ± 5.1 years. The mean age of Group A was 53 ± 6.4 years whereas the mean age of Group B was 55.1 ± 3.3 years. Group A included eight (53.3%) positive cases with a history of neuropathy while and Group B had six (40%). In Group A, six (40%) participants had a positive family history of RLS, while Group B had four (26.7%) (Table 1).
Table 1
Baseline demographic and clinical characteristics of participants
Between-group analysis showed no significant difference between groups A and B with regard to IRLS Total score, PSQI global or ESS total score (p > 0.05) at post-intervention analysis (Table 2). Although none of the between-group differences were statistically significant (p > 0.05), a clinically significant improvement was observed for the post-intervention mean IRLS total score (6.54), which was above the MCID value of 3–5 points. Likewise, the difference in ESS score (2.91) came close to, or reached, the MCID of 2–3 points, demonstrating a potentially significant change in daytime sleepiness. Nevertheless, the alteration in PSQI global scores (1.09) was less than the MCID (~ 3 points), suggesting a moderate clinical effect on sleep quality.
Table 2
Between-group comparison of IRLS, PSQI, and ESS scores
The within-group analysis identified significant changes in IRLS score (0.001), global PSQI score (0.001), and total ESS score (0.001) from baseline to post-intervention in both groups (p-value < 0.001; Table 3).
Table 3
Within-group comparison of IRLS, PSQI, and ESS scores
| Outcome variables | Level | Pre Mean (SD) | Post Mean (SD) | P-value | n (%) Achieving MCID |
|---|---|---|---|---|---|
| IRLS total score | Group A | 25.55 ± 9.62 | 20.18±8.88 | < 0.001* | 10/15 (66.7%) |
| Group B | 20.55 ± 9.13 | 16.64 ± 8.07 | < 0.001* | 9/15 (60.0%) | |
| PSQI global | Group A | 15.91 ± 3.4 | 12.00 ± 2.93 | < 0.001* | 9/15 (60.0%) |
| Group B | 14.09 ± 3.01 | 10.91 ± 3.11 | < 0.001* | 8/15 (53.3%) | |
| ESS total score | Group A | 12.82 ± 4.93 | 10.36 ± 4.22 | < 0.001* | 8/15 (53.3%) |
| Group B | 10.27 ± 5.06 | 7.45 ± 3.77 | < 0.001* | 9/15 (60.0%) |
The between-group analyses of the IRLS components were performed using the Mann Whitney U-test, for the non-parametric variables, and independent T-test, for the parametric variables. No significant intergroup differences (p ≥ 0.05) were observed for the following components at the post-intervention analysis: impact of RLS on everyday activity, mood difference, rating of RLS discomfort, need to move legs, relief with moving around, sleep disturbance, tiredness or sleepiness in the day, RLS symptoms or average RLS symptom severity, RLS severity as a whole (Table 4).
Table 4
Between-group analysis of individual components of International Restless Legs Syndrome Severity Scale
| Outcome variables | Level | Group A | Group B | U value | P-value | ||
|---|---|---|---|---|---|---|---|
| Mean Rank | Median (IQR) | Mean Rank | Median (IQR) | ||||
| Overall rating of RLS discomfort | Pre | 13.50 | 4(2) | 9.50 | 3(1) | 138.5 | 0.132 |
| Post | 13.50 | 2(2) | 9.50 | 2(1) | 38.0 | 0.125 | |
| Need to move legs | Pre | 13.36 | 4(2) | 9.64 | 3(2) | 40.00 | 0.160 |
| Post | 12.59 | 2(2) | 10.41 | 2(1) | 46.5 0 | 0.370 | |
| Relief with moving around | Pre | 13.09 | 3(1) | 9.91 | 3(2) | 43.00 | 0.224 |
| Post | 13.18 | 2(2) | 9.82 | 1(0) | 42.0 0 | 0.184 | |
| Sleep disturbance | Pre | 13.14 | 3(1) | 9.86 | 3(2) | 42.50 | 0.216 |
| Post | 12.45 | 2(2) | 10.55 | 2(1) | 50.0 | 0.461 | |
| Tiredness or sleepiness in the day | Pre | 13.32 | 3(2) | 9.68 | 2(2) | 40.50 | 0.172 |
| Post | 13.14 | 2(2) | 9.86 | 2(1) | 42.5 0 | 0.215 | |
| RLS severity as a whole | Pre | 13.77 | 3(1) | 9.23 | 2(1) | 35.0 | 0.086 |
| Post | 14.00 | 2(2) | 9.00 | 2(2) | 33.0 | 0.056* | |
| RLS symptoms | Pre | 13.77 | 3(1) | 9.23 | 2(1) | 35.0 | 0.084 |
| Post | 12.91 | 2(2) | 10.09 | 2(1) | 45.0 | 0.273 | |
| On average RLS symptoms severity | Pre | 13.64 | 1(1) | 9.36 | 2(2) | 37.0 | 0.104 |
| Post | 12.82 | 2(1) | 10.18 | 2(2) | 46.0 | 0.32 | |
| Impact of RLS | Pre | 12.45 | 2(2) | 12.00 | 2(2) | 66.8 | 0.183 |
| Post | 11.73 | 2(2) | 11.09 | 2(2) | 64.02 | 0.339 | |
| Mood disturbance | Pre | 12.45 | 2(3) | 11.82 | 2(1) | 56.43 | 0.221 |
| Post | 12.18 | 2(2) | 11.73 | 2(2) | 50.35 | 0.226 | |
The between-group analyses of the Pittsburgh sleep quality index components were performed using the non-parametric Mann-Whitney U-test. At the end of the intervention, statistically significant differences were noted in sleep quality (p = 0.003), sleep disturbance (p value = 0.003), and daytime dysfunction (p = 0.010); however, no significant differences in sleep latency, sleep duration, habitual sleep efficiency or sleep medication were found (p > 0.05; Table 5).
Table 5
Between-group comparison of individual components of the Pittsburgh Sleep Quality Index
| Outcome variables | Level | Group A | Group B | U value | P-value | ||
|---|---|---|---|---|---|---|---|
| Mean Rank | Median (IQR) | Mean Rank | Median (IQR) | ||||
| Sleep Quality | Pre | 12.0 | 3(1) | 11.0 | 3(1) | 55 | 0.67 |
| Post | 15.41 | 2(1) | 7.59 | 1(1) | 17.50 | 0.003* | |
| Sleep Latency | pre | 12.86 | 3(1) | 10.14 | 2(0) | 45.50 | 0.27 |
| post | 10.36 | 1(1) | 12.64 | 2(1) | 48 | 0.370 | |
| Sleep duration | Pre | 13.68 | 2(1) | 9.32 | 2(1) | 36.50 | 0.091 |
| post | 12.73 | 2(1) | 10.27 | 1(1) | 47 | 0.315 | |
| Habitual sleep efficiency | pre | 12.41 | 2(1) | 10.59 | 2(0) | 50.50 | 36.50 |
| post | 9.32 | 1(1) | 13.68 | 2(1) | 0.400 | 0.075 | |
| Sleep disturbance | pre | 11.50 | 2(0) | 11.50 | 2(0) | 60.50 | 1.00 |
| post | 8.00 | 1(0) | 15.00 | 2(1) | 22.00 | 0.003* | |
| Sleep medication | pre | 13.91 | 2(1) | 9.09 | 1(1) | 34.0 | 0.060 |
| post | 12.68 | 2(1) | 10.32 | 1(1) | 47.50 | 0.331 | |
| Daytime dysfunction | pre | 12.32 | 2(1) | 10.68 | 2(0) | 51.50 | 0. 484 |
| post | 14.68 | 2(1) | 8.32 | 1(1) | 25.0 | 0.010* | |
For between-group analyses of individual components of the Epworth sleepiness scale, the non-parametric Mann-Whitney U-test was applied. No significant differences in sleepiness were noted while reading, watching television, sitting inactive in a public place, in a car for an hour, while stopping in traffic, lying for rest in the afternoon, or sitting and talking (p > 0.05); however, a significant difference was noted for sitting quietly: mean rank 9.45 for Group A and 8.41 for Group B (p = 0.011; Figure 2).
DISCUSSION
This randomized controlled trial compares the effects of aerobic and strength training in individuals with diabetes mellitus experiencing Restless Legs Syndrome (RLS). The findings demonstrate that both aerobic and strength training significantly improved RLS severity, sleep quality, and daytime sleepiness; however, between-group comparisons revealed no statistically significant differences in these primary outcomes. Notably, subgroup analyses indicated that the strength training group showed significantly greater improvements in the sleep quality, sleep disturbance and daytime dysfunction subscales of the Pittsburgh Sleep Quality Index and the sitting quietly component in the Epworth Sleepiness Scale. Furthermore, although the differences were not statistically significant, the strength training group exhibited more favorable mean scores across all global measured outcomes compared to the aerobic training group.
The positive effects of aerobic training on reducing RLS severity have already been mentioned by several researchers, who conclude that aerobic training is an effective intervention. Our findings are in line with those of Mortazavi et al., which indicate that over a 16-week aerobic training program yielded statistically significant improvements (p < 0.05) in hemodialysis patients, with a mean difference of -5.5 (4.96) in RLS symptom severity observed between the first and final week of intervention20. Elsewhere, a 72-session course of aerobic training was found to have a better effect on RLS symptoms, as severity decreased from 24 at baseline to 15 after 36 sessions. At the end of 72 sessions, the score decreased further to 7 (p < 0.001)21. As RLS is associated with dysfunction in dopamine signaling, aerobic training is believed to relieve RLS by enhancing dopamine production and release.
Regarding sleep quality, a study by Amini et al investigated the impact of aerobic exercise for eight weeks on patients with RLS. Their findings revealed a significant improvement in sleep quality following aerobic exercises, which is in line with our present findings35. Reid et al.24 also highlighted the beneficial effects of aerobic exercise on sleep quality and daytime sleepiness and, subsequently, improvements in quality of life, among the elderly using PSQI and ESS: significant improvements wee noted in sleep duration (p = 0.04), sleep efficiency (p = 0.036), quality of sleep, sleep latency (p = 0.049), daytime dysfunction (p = 0.027), and daytime sleepiness (p = 0.02). It can be argued that this improvement in sleep quality and daytime sleepiness can be attributed to the increased oxygen uptake and energy production associated with regular aerobic exercises, as well as their beneficial effects on regulating circadian rhythms, releasing endorphins, and reducing anxiety and stress, thus creating a favorable mental state leading to a better sleep cycle35.
While strength training is another promising treatment option for improving sleep quality, its effect on RLS or RLS in diabetic patients has received little attention. However, an abundant body of evidence indicates that strength training may be effective in improving various health parameters. Our study is the first to compare the effect of both exercise programs on diabetes patients with RLS, and interesting results were found. The slightly superior outcomes observed in the strength training group, particularly in the daytime dysfunction component of the Pittsburgh Sleep Quality Index and the sitting quietly item of the Epworth Sleepiness Scale, may be attributed to the unique neurophysiological and metabolic effects of resistance exercises. Strength training is known to enhance neuromuscular activation, increase peripheral circulation, and modulate the dopaminergic pathway mechanisms implicated in the pathophysiology of RLS36. These findings are supported by those of Coban et al, which suggest that lower body-resistance training yielded significant improvements in RLS symptoms (p < 0.001) on the IRLSSG severity scale27.
The present study also found strength training to demonstrate a positive result on sleep quality; this is in line with Irandoust and Taheri, who report that the quality of sleep and psychomotor performance improved significantly in elderly men performing resistance training37. Similarly, a study of the effects of a strength training program on sleep quality and daytime sleepiness by Santiago et al. found both PSQI and ESS score to drop significantly over time, showing that strength training improves quality of sleep and increases total sleep duration, respectively37. Hugo Luca Corrêa et al.36 also indicate that resistance training can improve sleep quality, redox balance, inflammatory markers, and biomarkers of endothelial function. These benefits were also accompanied by increased muscle strength in both upper and lower limbs, suggesting that the incorporation of a resistance training program may have a positive impact on overall clinical status by addressing various health parameters.
However, the between-group analysis of IRLS, PSQI, and ESS revealed small to moderate effect sizes, suggesting that the clinical benefit of the interventions may be limited. This finding, combined with the lack of statistical significance in between-group comparisons, serves as a cautionary indicator and underscores the need for larger, adequately powered trials. Nevertheless, our findings are beneficial to researchers, clinicians and patients. For researchers, this study fills a gap in RLS management for diabetic neuropathy, offering new insights into non-pharmacological interventions. For clinicians, it provides evidence to support the use of exercise as a treatment option for improving RLS symptoms and sleep quality. For patients, the study highlights a practical, non-drug-based approach to managing RLS, which could improve quality of life and reduce reliance on pharmacological treatments.
This study also encountered several limitations that require consideration. Firstly, the intervention period of six weeks may have been insufficient to capture the full therapeutic potential of the exercise protocols; it is plausible that a longer intervention duration or extended follow-up might yield different or more significant outcomes. Secondly, ensuring patient availability multiple times a week under the conditions presented by the COVID-19 epidemic represented a considerable challenge; this also led to time constraints further affecting the strength of study, preventing the completion of a number of participants. Additionally, the findings may have been influenced by potential confounding factors such as medication use, pain characteristics which were not controlled. Finally, all participants were recruited from a single country of residence, which may affect the generalizability of results to broader populations. Future studies with larger samples, longer follow-up periods, and control of confounders are recommended to validate and extend these results.
CONCLUSIONS
This randomized controlled trial offers preliminary evidence that both aerobic and resistance training are beneficial in alleviating the symptoms of Restless Legs Syndrome (RLS) and enhancing sleep quality and daytime alertness in individuals with diabetes. Although no statistically significant differences were noted between the aerobic and resistance training groups, the latter exhibited modestly greater improvements in specific subdomains, in particular those associated with daytime dysfunction and sedentary wakefulness. These findings suggest a potential role for resistance training in addressing targeted aspects of RLS-related sleep disturbances. However, further research with larger sample sizes and extended intervention durations is necessary to substantiate these observations and explain the differential impact of exercise modalities.