INTRODUCTION
The body is covered by a three-dimensional network of fascia tissue: a liquid and solid mass that plays supporting, separating, protective, nutritional and communication roles; the tissue itself generally includes collagen fibers, elastin, and a large amount of water1. It is divided into three layers: the superficial layer (under the skin), the deep layer (dense fibrous tissue covering muscles), and the subserous fascia (which holds organs suspended in their cavities)1.
The fascia is also suitable for force transmission (FT), which results from myofascial release, muscle contraction, and stretching. Such transmission is not entirely confined to the muscle where it originates: it is estimated that approximately 30% of the tension is also transferred to adjacent tissues2. Recent cadaver studies suggest that muscle forces are transmitted not just longitudinally but also laterally, contributing significantly to homeostasis, musculoskeletal function, and pain management; in simpler terms, this tension is believed to occur within the deep fascia layer, encompassing intramuscular, intermuscular, and extramuscular pathways3. However, evidence for the existence of FT in living humans remains limited, and there is a need for a systematic analysis to address this gap.
Hence, it is possible that the muscles of the human body do not act as separate units. Rather, they are interwoven in specific myofascial chains, forming a network of systemic connections4. Myofascial chains allow forces generated by muscles to be transmitted to extramuscular structures not directly associated with that muscle3. The myofascial tissue that distributes and transmits forces has large numbers of mechanical receptors and plays an important role in coordinating movement and joint stability2. There is abundant evidence of morphological connectivity between different parts of the fascia, suggesting that fascia may be morphologically connected in other areas and can transmit force from one component to another2–5.
Again, while recent animal, cadaver, and laboratory experiments have shown that FT in fascia can play an important role in homeostasis, musculoskeletal function and pain, the evidence regarding these interactions in human studies remains fragmented, and a comprehensive review is required to consolidate these findings. Therefore, it is hypothesized that disturbances in the structural properties of fascia tissue, such as its thickness, flexibility and elasticity, can lead to increased pain, decreased ROM, and decreased tissue function5. Many researchers are interested in the importance of fascia in musculoskeletal disorders and its potential impact on muscle activity6.
Recent research has shown that fascia is the most innervated tissue in the body after the skin. It is extensively innervated by free nerve terminals and postganglionic sympathetic fibers and has a potential role in proprioception and pain perception6–7. Understanding more about how these interactions work should allow a better understanding of the pathology of inter alia chronic muscle problems and overuse syndromes8. It can also help explain the phenomenon where muscle contraction in one area is felt in distant areas of the body9. This relationship enhances the mutual feedback between muscles and fascia to better regulate tension and stretching10. However, the mechanisms underlying these interactions, especially in remote myofascial interventions (RMI), remain insufficiently explored, again underscoring the need for a systematic review to clarify these dynamics.
As tension in any part of a myofascial chain can be transferred to the entire chain through the fascia biotensegrity network, the body itself can be considered as a complete kinetic chain. Understanding how these chains interact in different parts of the body could provide an insight into various fields, including etiology, injury prevention, and therapeutic interventions11.
A thorough examination of the effects of myofascial release provides a fresh perspective on assessment, treatment, and athletic performance4. This approach enables more holistic intervention strategies, allowing a more comprehensive approach to addressing chronic pain and dysfunction rather than focusing solely on localized structures, especially in cases where movement restrictions affect specific regions of the body5. However, the mechanisms underlying these interactions, especially in remote myofascial interventions (RMI), remain insufficiently explored. Therefore, to address this gap in the existing literature, the aim of this study is to provide an overview of the current understanding of the effects of RMI on musculoskeletal health and functional performance based on a systematic review of recent literature.
MATERIALS AND METHODS
Search strategy
The present study was performed as a systematic review of RCT and cross-over trials that used RMI in humans. There was no restriction based on age or sex. This study has been registered in PROSPERO with registration number CRD42024589547. The Preferred Reporting Items for Systematic Reviews (PRISMA) guidelines were followed12. The following electronic databases were searched for eligible studies from inception to September 2024: PubMed, CINAHL, EMBASE, Scopus, and Web of Science.
While the databases were generally searched with free-text terms, in PubMed, MeSH terms were used to identify studies focused specifically on human interventions, viz. “myofascial release therapy”, “range of motion”, and “physical functional performance”. To ensure broader search coverage, the following keywords were also used: “remote myofascial release”, “anatomy trains”, “myofascial chains”, “fascial meridians release”, “myofascial release treatment”, and “treatment and myofascial release”. These terms were carefully selected to ensure comprehensive coverage across various databases, focusing on studies related to myofascial interventions, functional outcomes, and musculoskeletal health.
Eligibility criteria for studies
Type of study: Only RCT and cross-over trials in English were used. All trials included one or two experimental groups for intervention and a control group (CG) for comparison. Any other type of publication such as editorials, concepts and letters, were excluded, along with studies including patients with any neurological, psychiatric, and surgical disorders.
Participants: No limitations were employed regarding sex, age, race, financial status, or ethnicity.
Interventions: All included studies used RMI in the intervention group (IG), while the control groups (CG) received either placebo treatments or no exercise interventions. However, studies comparing one RMI technique with another were also included.
Conceptual Framework: Myofascial chains refer to interconnected muscle pathways that link the origin of one muscle to the insertion of another. These chains follow specific anatomical trajectories throughout the body, contributing to structural integrity and postural stability1.
Inclusion and exclusion criteria for the study: The inclusion criteria comprised the application of myofascial interventions, including one of the methods of release, stretching, and contraction in a specific area and checking its effect on other parts of the body; publication in English language; publication in a reputable Web Of Science journal; having the full text of the article; in addition, the implementation of the exercises (intensity, duration, repetition, and type) should be clear. The exclusion criteria included the following: review articles, local intervention, and the effect of the intervention on the opposite limb and outside the myofascial chain.
Data Collection
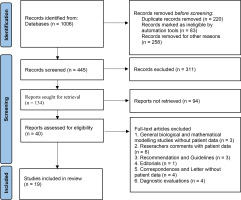
Study Selection: To minimize bias, the initial screening of articles from the international databases was conducted by two independent reviewers (BJ, RSH), which was followed by a random review of selected articles by a third, more experienced reviewer (RR). Disagreements were resolved by discussion or consultation with the third reviewer. The selection process involved an initial title review, followed by abstract screening, and full-text evaluation. Again, any disagreements were resolved through discussion or, if necessary, by exclusion. Studies that met the inclusion criteria were included for data extraction and quality assessment. The screening process is shown in Figure 1.
Due to the variability in study design, methodology, measurement tool, evaluated parameters, and test conditions within the identified papers, it was not possible to subject them to a full meta-analysis. Instead, a systematic review was performed, whose primary objective was to provide a comprehensive qualitative analysis of the available evidence. While a meta-analysis may be considered as a further step, should a sufficient number of comparable RCTs be identified, the main focus of this work is to present a qualitative synthesis of our findings.
Evaluation of the quality of studies: The quality of the selected studies was assessed with the PEDro scale, which is a reliable and valid measure often used in systematic reviews of RCTs. The PEDro scale assesses internal validity, statistical reporting, and external validity. The scale consists of 11 items, with each item receiving a positive sign (if correctly implemented) or a negative sign (if not). Points are awarded for each positive sign, and the total score ranges from 0 to 10. Scores of 9-10 are considered excellent, 6-8 are good, 4-5 are poor, and scores below 4 are very poor13.
Table 1
Evaluation of studies based on the PEDro scale. Yes (Y), No (N)
| Author, year | Eligibility criteria specification | Proper random allocation | Allocation was concealed | Similar at baseline | Subjects blinding | Therapists blinding | Assessor blinding | Initially allocated to groups | Intention to treat | Between group | Point estimate and variability | PEDro scale | Quality |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Aparicio et al.7 | Y | Y | N | Y | N | N | Y | N | N | Y | Y | 5 | Poor |
| Hyong et al.8 | Y | Y | N | Y | N | N | N | N | N | Y | Y | 4 | Poor |
| Grieve et al.9 | Y | Y | Y | N | N | N | Y | Y | Y | Y | Y | 7 | Good |
| Rodriguez et al.10 | Y | Y | Y | Y | N | Y | Y | Y | N | Y | Y | 8 | Good |
| Wilke et al.11 | Y | Y | N | Y | N | N | N | Y | Y | Y | N | 5 | Poor |
| Jung et al.14 | Y | Y | N | Y | N | N | N | Y | N | Y | Y | 5 | Poor |
| Joshi et al.15 | Y | Y | N | Y | N | N | Y | Y | Y | Y | Y | 7 | Good |
| Do et al.16 | Y | Y | N | N | N | N | N | Y | N | Y | Y | 4 | Poor |
| Jeong et al.17 | Y | Y | Y | Y | Y | N | Y | Y | N | Y | Y | 8 | Good |
| Cathcart et al.18 | Y | Y | N | Y | N | Y | Y | Y | Y | Y | Y | 8 | Good |
| Williams et al.19 | Y | Y | N | Y | Y | N | N | Y | N | Y | Y | 6 | Good |
| Martínez et al.20 | Y | Y | Y | Y | Y | N | Y | Y | N | Y | Y | 8 | Good |
| Kang et al.21 | Y | Y | Y | Y | N | N | Y | Y | Y | Y | Y | 8 | Good |
| Fauris et al.22 | Y | Y | Y | Y | N | Y | Y | Y | N | Y | Y | 8 | Good |
| Joshi et al.23 | Y | Y | N | Y | Y | N | N | N | N | Y | Y | 5 | Poor |
| Jeong et al.24 | Y | Y | Y | N | Y | N | Y | Y | Y | Y | Y | 8 | Good |
| Tamartash et al.25 | Y | Y | N | Y | N | N | N | Y | Y | Y | Y | 6 | Good |
| Ersin et al.26 | Y | Y | N | Y | N | Y | Y | Y | N | Y | Y | 7 | Good |
| Bali et al.27 | Y | Y | Y | Y | N | N | Y | Y | N | Y | Y | 7 | Good |
Information Extracted from the Studies: Data was extracted on sample size, mean age, intervention type for both IG and CG, the treatment arm in which each technique was used, the area of investigation, the myofascial chain targeted, and the conclusions. All extracted information is summarized in Table 2.
Table 2
Characteristics of the studies
| First Author | Testing method | Interventions | Subjects (N, sex) | Remote area/ Line | Conclusions |
|---|---|---|---|---|---|
| Aparicio et al.7 | Pre-test-Immediately-post-test Finger-floor test, SLRT Popliteal angle test | IG: 34 (22 ± 4y) SIT CG: 34 (22 ± 3 y) Joint articulation technique of the nose | N: 68 Hamstring shortness M/F: 47/23 | Hamstring elasticity Back myofascial Line | The suboccipital inhibition technique increases the flexibility of the hamstrings and may enable the therapist to affect more distant areas with this technique. |
| Hyong et al.8 | Pre-test-Immediately-post-test Cervical ROM (goniometer) Balance (Stability test) | IG: 30 (20.66 y) Hamstring and ankle dorsiflexion stretching CG: 30 (20.60y) Same treatment without ankle dorsiflexion | N: 60 Healthy students M/F: 26/34 | Cervical ROM SBL | Cervical ROM and balance immediately increased in the IG. |
| Grieve et al.9 | Pre-test-Immediately-post-test SAR The Beighton score | IG: 12 SMFR Plantar fascia CG: 12 No therapy | N: 24 Healthy volunteers (28 ± 11 y) M/F: 8/16 | Hamstrings Flexibility SBL | SMFR immediately increases the flexibility of the hamstrings and lower back in SBL. |
| Rodriguez et al.10 | Pre-test-immediately-post-test Vertical mouth opening PPT, suboccipital range of motion, SAR Lumbar forward bending. | IG: 30 (33 ± 9 y) Protocol CG + SIT CG: 30 (36 ± 12 y) Passive stretching Hamstring Neuromuscular technique over the masseter muscles | N: 60 Temporomandibular disorders M/F: 20/40 | Vertical mouth opening | The cumulative effect of combining local and distal techniques does not affect mouth opening in subjects with TMD. |
| Wilke et al.11 | Pre-test-immediately-post-test Ultrasonic 3D movement analysis system | IG1: 21 (38 ± 13) Remote stretching of gastrocnemius and hamstrings IG2: 21 (38 ± 13) Local stretching of the cervical CG: 21 (31±13) Inactive | N: 63 Healthy participants M/F: 31/32 | Cervical ROM SBL | Remote static stretching may immediately improve flexibility in cervical. The transmission of tension with myofascial pathways is a plausible explanation for the findings of this research. However, the influence of other structures such as peripheral nerves cannot be excluded. |
| Jung et al.14 | Pre-test-3 days-post-test SRT AROM PROM PPT (algometer) | G1: 22 (22 ± 2) SMFR suboccipital G2: 22 SMFR hamstring G3: 22 SMFR plantar regions | N: 22 Adult subjects M/F: 14/8 Cross-sectional design | Hamstring flexibility SBL | SMFR easily reduces pain and maintains flexibility, and can be performed anytime, anywhere. |
| Joshi et al.15 | Pre-test-2 weeks-post-test SAR Passive Knee Extension Angle | G1: 19 (23 ± 2 y) Hamstring Static Stretching G2: 20 (22 ± 1 y) MFR Plantar Fascia and Suboccipital G3:19 (21 ± 1 y) Combination | N: 58 Healthy people M/F: 16/42 | Hamstring flexibility SBL | A significant improvement in hamstring flexibility was observed in three groups, but more improvement was achieved in the consolidation group. |
| Do et al.16 | Pre-test-Immediately-post-test Toe Touch test and PSLR | IG: 15 (30±5 y) SMFR to the plantar fascia SG: 16 (23 ± 4 y) Passive mobilization of the ankle joint | N: 31 Healthy adults M/F: 19/12 | Hamstring and lumbar spine SBL | The results of this study showed that the release of plantar fascia is immediately effective for improving the flexibility of the lumbar spine and hamstrings. |
| Jeong et al.17 | Pre-test-Immediately/next day-post-test SLR CROM Popliteal angle Cranio-vertebral angle | G1: 10 (41 ± 13 y) SMI G2: 10 (39 ± 13 y) Cranio-cervical flexion exercise | N: 20 Neck pain M/F: | Hamstring ROM SBL | Both groups improve all of test results and are equally effective in immediately increasing hamstring flexibility, Cranio-vertebral angle, and CROM in subjects with neck pain. |
| Cathcart et al.18 | Pre-test-Immediately-post-test PPT (Algometer) ROM (Inclinometer) Interoceptive sensitivity (Biopac) | IG: 12 (23±7 y) MFR thoracic erector spinae (T6-T12) CG: 12 Lying supine and unilateral touch SG: 12 Not active touch | N: 12 Healthy subjects M/F: 7/5 | Lower limb and cervical | The increase in PPT and ROM (both local and distal) suggests that MFR may have caused a biomechanical change in tissue tension that increases tissue flexibility. |
| Williams et al.19 | Pre-test-Immediately-post-test SAR | G1: 15 (21 ± 1 y) MFR hamstring G2: 15 MFR plantar G3: 15 Both intervention | N: 15 Collegiate students M/F: 5/10 Crossover Study | Hamstring ROM SBL | SMFR may improve ROM, but one SMR technique does not appear to be superior to another. |
| Martínez et al.20 | Pre-test-immediately/next day-post-test SLR SAR Cervical flexion range of motion (goniometer) | IG: 34 (21 ± 1 y) MFR on posterior thigh CG:34 (20 ± 1 y) (Placebo) Forearm release | N: 68 Female university students | Hamstring and Cervical ROM SBL | MFR immediately increases hamstring ROM locally and neck ROM non-locally. The ROM of the neck decreased one day later. Therefore, more studies are needed. |
| Kang et al.21 | Pre-test-immediately/next day-post-test ROM of dorsiflexion Lunge angle Single Leg Balance Test were assessed, and a single leg balance test (SLBT) | G1: 20 (37 ± 7 y) 4-min SMI G2: 20 (36 ± 6 y) 8-min SMI G3: 20 (37 ± 5 y) 4-min sham-SMI G4: 20 (37 ± 5 y) 8-min sham-SMI | N: 80 Healthy adults M/F: 44/36 | ROM Ankle joint Lunge angle Balance | The SMI in 4 and 8 minutes, can be an effective tool for improving ROM, Lunge angle and balance. |
| Fauris et al.22 | Pre-test-0/5, 2, 5 and 10 min-post-test Modified SAR Dorsiflexion lunge test (DF-lunge) VAS | IG 1: 16 (25 ± 7 y) Plantar fascia IG 2: 18 (24 ± 5 y) Sural fascia IG 3: 15 (23 ± 5 y) Crural fascia IG 4: 15 (23 ± 4 y) Thoracolumbar fasciae IG 5: 15 (23 ± 6 y) Epicranial aponeurosis CG: 15 (26 ± 6 y) None | N: 94 Volunteers M/F: 52/42 | Hamstring flexibility SBL | The SBL may be considered a functional construct, and SMR to either segment can improve hamstring flexibility and ankle dorsiflexion. |
| Joshi et al.23 | Pre-test-4 weeks-post-test NPRS Modified Schober’s Test Oswestry Disability Index SF-36 questionnaire | G1: 15 (34 ± 9 y) SIT, interferential therapy and exercise G2: 15 (42 ± 10 y) Interferential therapy and exercise G3: 15 (38 ± 7 y) Exercise | N: 45 LBP M/F: - | Lumbar SBL | SIT combined with interferential treatment and exercises have better than interventional treatment and exercises alone in patients with LBP. |
| Jeong et al.24 | Pre-test-immediately/next day-post-test SLR Cranio-vertebral angle CROM | G1: 32 (35 ± 13 y) Static stretching hamstring G2: 32 (30 ± 9 y) PNF hamstring | N: 64 Neck pain M/F: 34/30 | CROM SBL | SLR, Cranio-vertebral angle, and CROM improved significantly within the groups after a one-session intervention. |
| Tamartash et al.25 | Pre-test-4 sessions-post-test Numeric Pain Scale Ultrasonography | IG: 16 (40 ± 5 y) MFR Crural fascia and hamstring CG: 16 (40 ± 4 y) MFR lumbar region | N: 32 Nonspecific LBP M/F: 16/16 | Changes in the Elastic Modulus and Pain Severity of lumbar. SBL | Remote MFR is effective in patients with chronic nonspecific LBP. |
| Ersin et al.26 | Pre-test-4 Weeks-post-test AKE Self-reported hamstring pain intensity was evaluated with a VAS | IG: 60 (26 ± 5 y) Home-based thoracic mobilization exercise for 4 weeks CG: 60 (25 ± 5 y) Active-assisted stretching of the hamstring | N: 120 Healthy subjects M/F: 37/83 | Hamstring flexibility SBL | Thoracic mobilization exercises may increase hamstring flexibility and decrease pain intensity during hamstring stretching exercises. |
| Bali et al.27 | Pre-test-4 Weeks-post-test PPT, Muscle strength, Cervical ROM, pain and Disability variables | IG: 17 (43 ± 8 y) MFR four fascia lines of the arm CG: 17 (46 ± 10) Exercise (stretching and strengthening) | N: 34 Cervical radiculopathy M/F: 10/24 | Cervical Four fascia lines of the arm | Cervical radiculopathy symptoms may improve after using myofascial release techniques. |
[i] AKE- Active Knee Extension, CG- Control Group, IG- Intervention Group, LBP- Low Back Pain, NPRS- Numerical Pain Rating Scale, PPT- Pain Pressure Threshold, PPSL- Passive Straight Leg Raise, SMFR- Self-Myofascial Release, SG- Sham Group, SAR- Sit-And-Reach, SLR- Straight Leg Raise, SBL- Superficial Back Line, SMI- Suboccipital Muscle Inhibition, VAS- Visual Analog Scale
Statistical Methods: As this study is a systematic review rather than a meta-analysis, no quantitative synthesis or effect size calculations were performed. The methodological differences and the variability in reported outcomes between studies were considered when interpreting the results.
RESULTS
A total of 1006 articles were identified in the search; however, only 40 were screened for eligibility. Of these, 19 studies met the inclusion criteria for the systematic review. The selection and rejection processes are given in Figure 1.
Quality of studies: The PEDro score ranged from 4 to 9. None of the studies had a score of less than 4. Each study mentioned eligibility criteria, and all performed between-group comparison analyses. All studies included random allocation of participants, but only eight described adequate concealment. Baseline comparisons were reported in 16 studies. Blinding of subjects and therapists was not performed in most studies due to the nature of the interventions, but 12 studies used blinded outcome assessment (Table 2).
Characteristics of subjects: The included studies comprised a total of 1030 subjects, with an age range of 18 to 60 years; the minimum number of samples was 12 people18 and the maximum number was 12026. One study only included women20 and another did not specify sex23. The mean PEDro score was 6.52, indicating that the studies were of high quality; none of the studies had a low rating.
Interventions: The described interventions included release, stretching, inhibition and mobilization by the individual and the therapist. The duration of interventions ranged from 30 seconds to four minutes in one to three sets. The evaluation in all studies was pre-test and post-test. The intervention time ranged from immediate, up to one hour, two weeks and four weeks. Finally, all but three studies worked on the SBL10,21,27.
Outcome measures and results: The results were obtained after applying hamstring flexibility interventions in 10 studies21,23,26,27,28,29,30,31,33,16. In addition, five studies examined the cervical spine,2,25,29,30,37 two studies the lumbar spine23,25 and one study concerned the vertical opening of the mouth10. All but five studies were conducted on healthy subjects11,16,24,29,35. The following variables were measured: ROM (all studies), pain, PPT, balance, subcutaneous fascia tissue / superficial fascia for calculating the elastic modulus, and interceptive sensitivity.
Five studies21,24,29,33,35 using the suboccipital muscle inhibition technique (SIT) in the posterior chain showed an increase in the ROM in the remote area, including the hamstrings and ankles, as well as improved balance. Five studies23,26,27,30,31 achieved increased range of ROM in the hamstrings and cervical spine following MFR treatment of the plantar fascia with a massage ball and wooden roller. Five studies2,25,29,30,37 also showed an increase in ROM in the cervical by stretching and MFR treatment of the hamstrings. Three studies17,23,25 yielded an increase in the ROM in the cervical spine and hamstrings, as well as a reduction in pain and an improvement in the elastic modulus following stretching and RMI in the back of the leg. One study24, showed improved ROM in the hamstrings and neck following thoracic mobilization and RMI.
To allow easier comparison of the outcomes between studies, to provide greater transparency, and to improve the presentation of quantitative results, statistical data (such as means, standard deviations, and p-values) from the included studies is provided in a clear and concise manner in Table 3. It serves as a quick reference for readers to understand the variation and effectiveness of the interventions across studies.
Table 3
Summary of Study Outcomes and Statistical Data
| Study ID | Outcome | Time | Mean±SD (IG) | Mean±SD (CG) | Change (IG) | p-value | ||
|---|---|---|---|---|---|---|---|---|
| Per-test | Post-test | Per-test | Post-test | |||||
| Aparicio et al.7 | SLR test-R | I | 59.22 ± 12.16 | 65.11 ± 12.68 | 64.50 ± 9.21 | 64.76 ± 9.77 | 5.89° | 0.001 |
| SLR test-L | I | 58.38 ± 9.96 | 63.41 ± 9.58 | 62.29 ± 8.40 | 63.02 ± 7.48 | 5.50° | 0.001 | |
| Popliteal angle-R | I | 31.97 ± 10.22 | 27.83 ± 10.07 | 27.29 ± 10.31 | 26.44 ± 9.58 | 4.1° | 0.05 | |
| Popliteal angle-L | I | 32.25 ± 8.34 | 27.08 ± 9.98 | 28.23 ± 9.30 | 27.67 ± 9.23 | 5.2° | 0.001 | |
| Hyong et al.8 | Neck flexion | I | 50.06 ± 11.09 | 55.13 ± 10.12 | 54.13 ± 9.97 | 53.53 ± 9.75 | 5.07° | 0.001 |
| Neck extension | I | 58.86 ± 9.68 | 64.26 ± 10.04 | 60.33 ± 8.08 | 61.20 ± 7.56 | 5.4° | 0.001 | |
| Stability test | I | 15.27 ± 4.36 | 13.56 ± 3.78 | 14.68 ± 4.42 | 13.82 ± 4.00 | -1.71 | 0.001 | |
| Grieve et al.9 | SRT test | I | 17.92 ± 11.62 | 20.33 ± 11.37 | 21.58 ± 10.62 | 22.42 ± 10.37 | 2.41 cm | 0.03 |
| Rodriguez et al.10 | SRT test | I | 20.32 ± 7.24 | 21.93 ± 8.25 | 21.26 ± 6.46 | 22.51 ± 6.22 | 1.61 cm | 0.003 |
| Vertical-mouth opening | I | 38.29 ± 7.57 | 40.12 ± 6.04 | 39.72 ± 7.13 | 39.72 ± 9.02 | 1.83 cm | 0.057* | |
| ROM LUMB | I | 96.78 ± 29.51 | 103.36 ± 27.43 | 109.83 ± 33.70 | 99.28 ± 36.52 | 6.58° | 0.009 | |
| Wilke et al.11 | Sagittal plane | I | 4° | 0.03 | ||||
| Transversal plane | I | 3.5° | 0.02 | |||||
| Frontal plane | I | 9° | 0.001 | |||||
| Jung et al.14 | SRT (Suboccipital) | 3-D | 22.89 ± 9.44 | 22.89 ± 9.44 | 4.06 cm | 0.033 | ||
| SRT (Hamstring) | 3-D | 22.61 ± 10.05 | 26.47 ± 9.27 | 3.86 cm | 0.033 | |||
| SRT (Plantar) | 3-D | 22.08 ± 10.11 | 25.94 ± 9.85 | 3.86 cm | 0.033 | |||
| Joshi et al.15 | SRT (Stretching) | 2-W | 31.7 ± 7.8 | 36.9 ± 8.2 | 5.2 cm | 0.004 | ||
| SRT (remote) | 2-W | 32.6 ± 6.5 | 35.6 ± 7.3 | 3 cm | 0.007 | |||
| SRT (combination) | 2-W | 32.4 ± 8.5 | 39.1 ± 7.7 | 6.7 cm | 0.006 | |||
| Do et al.16 | Toe Touch test | I | 17.88 ± 6.98 | 13.22 ± 6.91 | 17.76 ± 9.59 | 16.28 ± 8.37 | -4.66 cm | 0.001 |
| SLR-R | I | 45.27 ± 5.99 | 53.73 ± 8.87 | 49.93 ± 7.46 | 48.43 ± 8.64 | 8.46° | 0.001 | |
| SLR-L | I | 45.6 ± 7.10 | 54.13 ± 8.12 | 51.43 ± 10.25 | 52.06 ± 10.20 | 8.53° | 0.001 | |
| Jeong et al.17 | SLR (SMI) -R | I | 72.8 ± 6.7 | 77.2 ± 4.5 | 4.4° | 0.005 | ||
| SLR (SMI)- L | I | 75.8 ± 4.0 | 83.4 ± 5.9 | 7.6° | 0.008 | |||
| SLR (CCFE)- R | I | 69.1 ± 15.9 | 78.5 ± 13.5 | 9.4° | 0.008 | |||
| SLR (CCFE)- L | I | 69.9 ± 14.8 | 79.3 ± 12.5 | 9.4° | 0.01 | |||
| Cathcart et al.18 | PPT-L | I | 0.24 | 0.04 | ||||
| PPT-R | I | 0.18 | 0.1 | |||||
| ROM | I | 5.72° | 0.01 | |||||
| Williams et al.19 | SRT (foot) | I | 31.06 ± 9.91 | 32.19 ± 10.2 | 1.13 cm | - | ||
| SRT (hamstring) | I | 30.49 ±11.03 | 32.76 ± 11.34 | 2.27 cm | - | |||
| SRT (both) | I | 29.19 ± 12.60 | 31.10 ± 13.15 | 1.91 cm | .079* | |||
| Martínez et al.20 | SRT | I | 20.02 ± 3.93 | 22.52 ± 3.87 | 21.41 ± 3.23 | 21.74 ± 3.73 | 2.5 cm | 0.001 |
| SLR-R | I | 75.02 ± 9.97 | 82.17 ± 11.65 | 78.32 ± 10.34 | 79.76 ± 9.19 | 7.15° | 0.001 | |
| SLR-L | I | 78.32 ± 10.73 | 84.55 ± 10.89 | 79.67 ± 9.55 | 80.35 ± 9.04 | 6.23° | 0.001 | |
| CROM | I | 42.73 ± 6.76 | 47.44 ± 6.39 | 45.61 ± 5.48 | 45.61 ± 6.23 | 4.71° | 0.001 | |
| Kang et al.21 | AROM- 4 min SIT | I | 23.0 ± 5.1 | 29.5 ± 5.6 | 25.3 ± 4.9 | 25.6 ± 4.9 | 6.5° | 0.001 |
| AROM- 8 min SIT | I | 25.0 ± 4.1 | 30.6 ± 5.2 | 25.0 ± 5.8 | 25.2 ± 5.8 | 5.6° | 0.001 | |
| LA- 4 min SIT | I | 46.1 ± 6.9 | 51.2 ± 6.7 | 47.4 ± 5.6 | 47.2 ± 5.9 | 5.1 | 0.001 | |
| LA- 8 min SIT | I | 48.4 ± 5.2 | 52.6 ± 5.8 | 48.4 ± 5.2 | 52.6 ± 5.8 | 4.2 | 0.001 | |
| SLBT- 4 min SIT | I | 81.7 ± 31.1 | 109.9 ± 42.6 | 80.1 ± 45.4 | 86.3 ± 44.5 | 28.2 | 0.001 | |
| SLBT- 8 min SIT | I | 72.7 ± 33.4 | 119.5 ± 45.3 | 91.8 ± 34.5 | 91.6 ± 35.2 | 46.8 | 0.001 | |
| Fauris et al.22 | SRT | 10m | 5.22cm | |||||
| DF-lunge | 10m | 0.65° | ||||||
| Joshi et al.23 | NPRS (SIT) | 4-W | 5.53 ± 0.9 | 0.23 ± 0.44 | 5.3 | 0.001 | ||
| Lumbar Flexion (SIT) | 4-W | 4.95 ± 0.41 | 5.76 ± 0.29 | 0.81° | 0.001 | |||
| Lumbar Extension (SIT) | 4-W | 1.23 ± 0.35 | 1.52 ± 0.25 | 0.29° | 0.046 | |||
| ODI (SIT) | 4-W | 30.90 ± 11.16 | 3.54 ± 4.73 | -27.36 | 0.001 | |||
| SF-36 (SIT) | 4-W | 58.45 ± 10.51 | 77.97 ± 9.94 | 19.52 | 0.001 | |||
| Jeong et al.24 | SLR (SS) | I | 71.8 ± 7.9 | 84.2 ± 9.9 | 12.4° | 0.007 | ||
| SLR (PNF) | I | 72.0 ± 2.9 | 80.3 ± 4.9 | 8.3° | 0.005 | |||
| CVA (SS) | I | 42.3 ± 9.9 | 47.8 ± 8.0 | 5.5° | 0.02 | |||
| CVA (PNF) | I | 45.0 ± 8.2 | 52.0 ± 7.9 | 7° | 0.007 | |||
| CROM (SS) | I | 50.5 ± 4.2 | 53.1 ± 3.9 | 2.6° | 0.008 | |||
| CROM (PNF) | I | 53.5 ± 5.4 | 54.9 ± 4.6 | 1.4° | 0.06* | |||
| Tamartash et al.25 | NPRS | 4-S | 5.44 ± 0.62 | 3.12 ± 0.88 | 5.50 ± 0.51 | 3.37 ± 0.80 | -2.32 | 0.001 |
| Elastic Modulus | 4-S | 81.11 ± 25.15 | 38.81 ± 19.41 | 64.93 ± 34.30 | 31.06 ± 11.90 | 42.3 | 0.001 | |
| Ersin et al.26 | AKE | 4-W | 27.33 ± 8.8 | 17.01 ± 7.81 | 29.32 ± 7.72 | 22.93 ± 8.76 | 10.32° | 0.05 |
| VAS | 4-W | 43.33 ± 22.26 | 30.22 ± 11.45 | 50.06 ± 26.55 | 43.45 ± 15.87 | -13.11mm | 0.05 | |
| Bali et al.27 | Cervical Flexion | 4-W | 4.27 ± 1.75 | 5.28 ± 1.19 | 4.88 ± 2.60 | 3.85 ± 2.23 | 1.01° | 0.013 |
| NDI | 4-W | 21.56 ± 7.59 | 6.94 ± 2.01 | 16.02 ± 6.66 | 15.56 ± 8.14 | 14.62 | 0.001 | |
| VAS | 4-W | 6.88 ± 2.93 | 1.14 ± 1.46 | 5.87 ± 2.45 | 4.28 ± 2.65 | -5.74 cm | 0.001 | |
[i] AROM- Active Range Of Motion, I- Immediate, CROM- Cervical Range Of Motion, CCFE- Cranio-Cervical Flexion Exercise, CVA- Cranio-Vertebral Angle, D- Days, DF-lunge- Dorsiflexion Lunge Test, KEA- Knee Extension Angle, LA- Lunge Angle, NDI- Neck Disability Index, NRS- Numerical Pain Rating Scale, ODI- Oswestry Disability Index, PPT- Pain Pressure Thresholds, PNF- Proprioceptive Neuromuscular Facilitation, S- Sessions, SRT- Sit and Reach Test, SLBT- Single Leg Balance Test, SF-36 Questionnaire, SS- Static Stretching, SIT- Suboccipital Muscle Inhibition Technique, VAS- Visual Analog Scale, W- Weeks, *(p < 0.05)
DISCUSSION
A systematic review was performed of studies evaluating the effects of RMI along muscle chains on hamstring flexibility, cervical spine, lumbar spine, and vertical opening of the mouth. Our findings offer valuable insights into the effectiveness of various techniques associated with the fascia, including SIT, myofascial release, and stretching exercises. The studied interventions were evaluated based on their impact on ROM, pain, balance, and tissue elasticity. SIT was found to significantly increase ROM in remote areas such as the hamstrings and ankles. This suggests that SIT can have a systemic effect, potentially due to neural or fascial connections that influence distant muscle groups17,21.
Myofascial release was consistently shown to improve ROM in the hamstrings and cervical regions, reduce pain, and enhance tissue elasticity29,30,31,32. The technique appears to be effective in both immediate and short-term applications, with some studies indicating superior results compared to stretching alone33. Additionally, myofascial release combined with other modalities, such as passive stretching, has been found to obtain significant improvements in plantar pressure and popliteal angle, indicating enhanced overall flexibility and functional performance32. Stretching exercises, particularly static stretching, were effective in increasing hamstring flexibility and improving balance33,34. However, the combination of stretching with other techniques, such as proprioceptive neuromuscular facilitation or myofascial release, often yielded better results6,35. Stretching also showed benefits in reducing perceived muscle tightness and improving gait biomechanics, which are crucial for athletic performance and injury prevention36.
The main finding regarding the use of RMI was the increase in ROM in other parts of the body, and most studies focused on the superficial back line (SBL). This line is stretched in the posterior part of the body, from the plantar fascia to the frontal part of the head, and its function is to support the body in a vertical position and bend forward. The existence of this chain has been proven in multiple studies37. For example, Jeong et al. demonstrated that increased hamstring tension and shortening could contribute to neck and shoulder pain due to SBL connections17.
Myofascial techniques have been found to result in improvements in ROM, which also suggest systemic effects beyond localized interventions38. Burk et al.39 attribute the increase in ROM associated with myofascial release to neurophysiological and mechanical responses, such as the piezoelectric effect, viscoelastic tissue changes, and mechanoreceptor activation; however, Burk et al.39 also acknowledge that the piezoelectric effect has not been validated in vivo. Although RMI has been demonstrated to have acute effects on muscle chains, the duration of these effects remains unclear. The researchers suggest that the impact lasts between 10 and 30 minutes40.
The muscles forming the human body are arranged in such a way that they form myofascial chains, with the end of one muscle connected to the beginning of another38. Mechanically, these effects are mediated by sarcomere contractions, extracellular matrix adaptations, and mechanoreceptor stimulation4,39,41. Studies by Wilk et al.42 have confirm that tension transfer occurs first within muscle groups and then between adjacent muscles along myofascial pathways. In addition, some prominent myofascial chains have been confirmed in post mortem studies at the macroscopic level39.
Several limitations were identified in the included studies, such as small sample sizes, limited participant diversity, short-term outcome measurements without follow-up assessments and lack of standardization in intervention protocols (e.g., duration, frequency, and pressure applied). In addition, some studies had lower PEDro scores and hence may be subject to selection bias. Furthermore, some research was prone to uncontrolled confounding factors, such as mental and nutritional influences, which may impact intervention efficacy. Addressing these limitations is essential to enhance the reliability and applicability of future research.
Nevertheless, the data indicates that RMI may be particularly beneficial for individuals with limited ROM due to muscular tightness, athletes aiming to enhance flexibility and prevent injury, and patients with postural dysfunctions or chronic pain syndromes. However, further studies with standardized methodologies, larger sample sizes, and long-term follow-up assessments are needed to confirm these findings.
Furthermore, it seems that RMI may offer advantages over conventional stretching and massage. The data indicates that it can achieve more significant increases in ROM compared to static stretching alone33 and more profound effects when combined with stretching or proprioceptive neuromuscular facilitation6,35. It also offers additional benefits for pain reduction and functional performance40. Future studies should directly compare RMI with standard myofascial release and traditional exercise therapy to establish its relative effectiveness.
This systematic review highlights the potential of RMI for improving ROM, reducing pain, and enhancing musculoskeletal function. The findings suggest that targeting myofascial chains may provide a novel approach for rehabilitation and performance enhancement. However, methodological inconsistencies, small sample sizes, and short-term focus limit the generalizability of these results. Future research should address these limitations to strengthen the evidence base for RMI applications in clinical and athletic settings.
CONCLUSIONS
The findings of this systematic review suggest that myofascial chains play a significant role in the transmission of mechanical forces (FT) beyond local muscle groups, influencing flexibility and range of motion (ROM) in distant body regions. The included studies support the idea that interventions targeting specific points within these chains can induce systemic effects, likely mediated by mechanoreceptor activation, autonomic nervous system modulation, and changes in fascial hydration and elasticity.
However, fascial function and the outcomes of myofascial interventions are influenced by several factors, such as tissue directionality, age, physical activity level, emotional state and the presence of pathological conditions. These variables thus present a challenge in standardizing research methodologies and interpreting findings across different populations.
Despite evidence supporting the presence of force transmission in myofascial chains, the extent, mechanisms, and long-term clinical significance of these effects remain areas for further investigation. Future studies should focus on identifying optimal intervention protocols, assessing long-term effects, and improving methodological consistency to enhance the applicability of myofascial interventions in both rehabilitation and athletic performance contexts.