Introduction
Colorectal malignancies occur frequently and are the fourth most common cause of malignancy-related mortality in the world. It is estimated that 2.2 million new diagnoses and 1.1 million mortality cases will develop annually by 2030 [1]. Laparoscopy has recently emerged as a popular technique around the world due to its successful oncological outcomes in colorectal surgery similar to those of open surgery as well as its remarkable advantages. The description and standardization of total mesorectal excision (TME) and the use of neoadjuvant chemoradiotherapy (CRT) have led to dramatic improvement in oncological outcomes [2–4]. However, whether complete splenic flexure mobilization (SFM) is required remains a controversial issue and there are numerous approaches regarding the performance of this procedure [5–8]. The primary aim in the performance of laparoscopic complete SFM is to achieve adequate oncological resection and to ensure a tension-free anastomosis with a good blood supply. A complete SFM consists of the division of the splenocolic, phrenicocolic, gastrocolic, and pancreaticomesocolic ligaments [9–11].
Splenic flexure mobilization, whether it be open or laparoscopic, is the most demanding part of laparoscopic colorectal surgery with complex technical details. Accordingly, SFM has been shown to increase operative time and the incidence of splenic injury and other complications [12–14]. Splenic injury is a leading complication caused by SFM with a reported incidence of 0.5–8% and has been shown to have adverse effects on oncological outcomes. The incidence of splenic injury is remarkably lower in laparoscopic surgery compared to open surgery [15–18]. The procedure can be performed using a lateral-to-medial, medial-to-lateral, anterior, or combined approach. However, both SFM and the mobilization of the left colon are mostly performed using the medial-to-lateral approach. This approach enables immediate identification of the embryological plane and renders the dissection fast and safe. Moreover, after ligation of the vascular pedicle, tumor manipulation is minimized and the mesocolon can be rapidly freed from the retroperitoneal space without bleeding [19, 20].
Aim
In this study, we aimed to investigate the effect of SFM performed with a medial-to-lateral and superior-to-inferior approach on early clinical outcomes in laparoscopic resection of rectal cancer.
Material and methods
Study design and patient selection
The retrospective study included patients who underwent surgical treatment due to a diagnosis of rectal adenocarcinoma in our clinic between January 1, 2017 and December 31, 2018. The study was approved by the local ethics committee. Patients who underwent emergency surgery, open surgery, abdominoperineal resection, and partial SFM were excluded from the study. Each patient underwent oral and intravenous contrast-enhanced abdominal and pelvic computed tomography (CT), thoracic CT, and pelvic magnetic resonance imaging (MRI) for tumor staging. Patients who had an early-stage tumor and were planned for transnasal excision underwent endorectal ultrasonography. Based on the indications, neoadjuvant CRT was performed according to current guidelines. The anastomotic line is below the pelvic reflection, or in case of neoadjuvant treatment, protective loop ileostomy is performed.
Data collection
Data were collected via patient registries, hospital database records, surgical notes, and final pathological reports. Demographic characteristics, neoadjuvant CRT performance, operative time, intraoperative blood loss and complications, postoperative complications, and length of hospital stay were recorded for each patient. Postoperative complications were classified based on the Clavien-Dindo classification [21]. Anastomotic leakage was defined as gas or fecal discharge from the incision line, vagina, or the drain tract, extravasation of contrast material and air bubbles around the anastomosis verified by rectal contrast-enhanced CT, and the presence of an anastomotic defect verified by laparotomy or rectal examination [22]. Evaluation of anastomotic stenosis was performed based on the presence of clinical suspicion. Anastomotic stenosis was defined as the inability to pass a 12-mm diameter sigmoidoscope through the anastomosis within 6 months after the surgery. Tumor staging was performed according to the Union for International Cancer Control-American Joint Committee on Cancer (UICC-AJCC) TNM Classification System 7th Edition [23]. Length of hospital stay was defined as the time from the initiation of surgery to hospital discharge.
Surgical technique
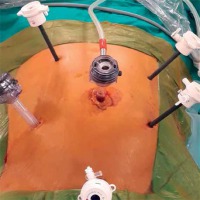
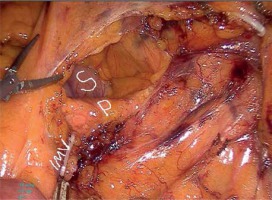
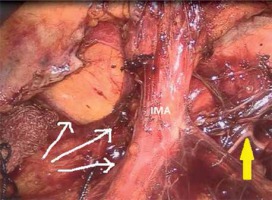
A camera port was entered in the supraumbilical site. Under direct view, five surgical ports were created, including a 12-mm port in the lower right, a 5-mm port in the upper right, a 5-mm port in the upper left, and a 5-mm in the lower left quadrant and a 12-mm port in the suprapubic region (Photo 1). Following the surgical exploration of the abdomen, the patient was placed in a right-sided Trendelenburg position with the small bowel placed in the upper right quadrant. Dissection was initiated by retracting the descending colon and sigmoid colon and opening a window in the mesocolon lateral to the ligament of Treitz. The inferior mesenteric vein (IMV) was clipped and divided at the lower border of the pancreas. The middle colic artery (MCA) was preserved intact and the omental bursa was opened by dissecting the pancreatico-mesocolic ligament inferiorly from the upper border of the pancreas (Photo 2). The mesocolon was excised up to the level of the splenic hilum and was continued along the embryological fascial plane upward to the border of the inferior mesenteric artery (IMA), laterally along the retroperitoneal area up to Toldt’s fascia. Subsequently, the IMA was clipped and divided (Photo 3). The mesocolon was released cranially to caudally and the presacral area was reached. Lateral peritoneal adhesions were dissected and the omentum was divided up until 2/3 of the way along the transverse colon. The SFM procedure was completed by dividing all the splenocolic, phrenicocolic, gastrocolic, and pancreaticomesocolic ligaments. The surgical procedure was continued according to the standard TME procedure.
Results
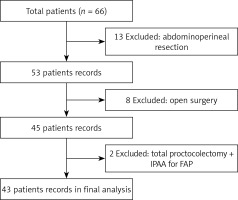
The study reviewed medical records of 66 patients who were operated on due to rectal cancer in our clinic between January 2017 and December 2018. Of these, 23 patients who underwent abdominoperineal resection, open surgery, or total colectomy due to adenomatous polyposis of the colon were excluded and thus a total of 43 patients were included in the study (Figure 1). The patients included 26 (60.5%) men and 17 (39.5%) women with a mean age of 58.2 ±13.9 (range: 30–87) years. Comorbidities were present in 15 (34.8%) patients, including diabetes mellitus (DM), hypertension, ischemic heart disease, and chronic obstructive pulmonary disease (COPD). Thirty (69.8%) patients had an ASA (American Society of Anesthesiologists) score of II. Table I presents the demographic, operative, and pathological characteristics of the patients. Mean operative time was 271 ±50 min and mean blood loss was 144 ±83 ml. Two (4.7%) patients underwent simultaneous metastasectomy for peripheral metastasis in the liver. A protective ileostomy was created in 37 (86%) patients. Six (14%) patients had stage 0 disease, of whom 5 patients showed a pathological complete response following CRT and the remaining one patient was diagnosed with tubulovillous adenoma with low-grade dysplasia.
Table I
Demographic, operative and pathological results of patients
No intraoperative splenic, pancreatic, ureteral, or adjacent organ injury occurred in any patient. Ileus was the most common postoperative complication (n = 6; 14%), followed by bleeding (n = 4; 9.3%), sepsis (n = 1; 2.3%), pulmonary embolism (n = 1; 2.3%), atelectasis (n = 1; 2.3%), urinary retention (n = 1; 2.3%), evisceration (n = 1; 2.3%), pancytopenia (n = 1; 2.3%), bladder fistula (n = 1; 2.3%), and anastomotic leakage (n = 1; 2.3%) (Table II). The patient with anastomotic leakage was receiving bevacizumab therapy due to metastatic disease prior to the surgery. The patient was operated on 7 weeks after the cessation of the bevacizumab therapy and the patient underwent low anterior resection, partial bladder resection, and metastasectomy for liver metastasis. After resuming the bevacizumab therapy postoperatively, the patient developed hypertension, wound site infection, sepsis, and bladder fistula in addition to anastomotic leakage. The patient was treated using conservative methods. Mean length of hospital stay was 9.3 ±4.3 (range: 5–28) days and no early mortality occurred in any patient. No anastomotic stenosis was observed in any patient in the follow-up period.
Discussion
Splenic flexure mobilization is a complex part of laparoscopic colorectal procedures. A safe SFM not only achieves a tension-free colonic segment with a good blood supply but also increases compliance with oncological principles [9–11, 24]. The morbidity and adverse oncological outcomes associated with colorectal anastomotic leakage are well known. For this reason, the surgeon must pay appropriate attention to preventable risk factors for anastomotic leakage to create a safe anastomosis. Moreover, they should also recognize that the incidence of anastomotic leakage can be reduced by SFM [9, 11, 25–27]. However, there are some studies in the literature advocating that SFM should be performed in selected cases due to its learning curve and complexity, prolonged operative time, and the increased risk for splenic, pancreatic, and adjacent organ injury [6, 7, 12–14, 16]. Ferrara et al. reported that SFM increased the operative time, the incidence of conversion to open surgery, and the complexity of the operation. The authors also noted that SFM had no superiority in terms of postoperative complications and oncological outcomes [14]. In a recent systematic review, Nowakowski et al. reported that SFM led to a 3.2-fold increase in the operative time and a 3-fold increase in the incidence of anastomotic leakage compared to the patients who did not undergo SFM [7]. Inarguably, the absence of prospective, randomized, and controlled studies on SFM remains an important issue.
Splenic flexure mobilization is often required for the creation of a tension-free anastomosis with good blood supply in patients undergoing ultra-low anterior resection for middle and lower rectal cancer. Moreover, SFM may even become mandatory in patients requiring a colonic pouch [28]. Laparoscopy is highly advantageous since it allows a large dissection on the embryological plane without increasing the incidence of adjacent organ injury. Splenic flexure mobilization, on the other hand, increases the usable length of the colonic segment [8, 10, 11]. Mouw et al. [10] reported that SFM enabled adequate lymph node dissection and an adequate distal resection margin. The literature indicates that there are a number of risk factors associated with anastomotic leakage in low rectal cancer, including male gender, ASA score of ≥ III, neoadjuvant CRT, coronary artery disease, prolonged operative time, and intraoperative blood transfusion [9, 25]. Kim et al. [9] reported that SFM is a highly valuable factor in the reduction of morbidity associated with anastomotic leakage and suggested that SFM should be used in laparoscopic resection of rectal cancer. It is recommended to perform it as the standard stage of surgical training [24].
The literature indicates that there is no standardized approach for laparoscopic SFM. However, the medial-to-lateral approach has a shorter duration compared to the lateral approach, also causing no additional complications. By using the medial-to-lateral approach, dissection of the mesocolon following high vascular ligation can be rapidly performed with no bleeding. On the other hand, the higher magnification and high-definition resolution provided by laparoscopy allow better visualization of embryological and anatomic planes [8, 29]. Benseler et al. [5] reported that the lateral approach for SFM was associated with higher complication rates compared to the medial and anterior approaches. The authors also noted that the anterior approach had the lowest intra- and post-operative complication rates [5]. While employing the medial-to-lateral approach, surgeons often prefer to continue the dissection by preserving the ureter after isolating and dividing the IMA. In this way, however, the IMA is highly likely to be injured since it has a short origin arising from the aorta, it is difficult to isolate the IMA due to dense mesocolic fat, and the IMA is adjacent to autonomic nerve structures. For these reasons, the IMV, which can be visualized more easily, can be used as an anatomical landmark providing convenient points of reference for adjacent soft tissue structures, thereby facilitating the procedure [19].
In our clinic, SFM has been routinely performed in laparoscopic resection of rectal cancer since 2016. The primary step in our procedure consists of IMV ligation followed by the freeing of the pancreaticocolic ligament inferiorly from the upper border of the pancreas to the splenic hilum. In the second step, the mesocolon is freed caudally up to the margin of the IMA, followed by the ligation of the IMA. The dissection of the mesocolon is continued up to the presacral space in a superior-to-inferior and medial-to-lateral fashion. We consider that this procedure is advantageous in several ways: i) an appropriate anatomical plane is reached through the continuation of the dissection along IMV lateral to the ligament of Treitz followed by the ligation of the IMV at the lower border of the pancreas, ii) the dissection extending from the upper border of the pancreas to the splenic hilum is facilitated, iii) the superior-to-inferior dissection technique leads to a lower risk of adjacent organ injury as the dissection begins in a site distant from the ureter and gonadal vascular structures, and iv) the autonomic neurogenic structures can be preserved as the IMA can be visualized better in the site with dense mesocolic fat where it originates from the aorta. On the other hand, we believe that complete SFM reduces the complications, particularly those related to anastomosis. In our study, only 1 patient developed anastomotic leakage, which might have occurred secondary to bevacizumab therapy. However, no sign of anastomotic tension was noted intraoperatively and no anastomotic stenosis occurred in any patient during the follow-up period.
Our study was limited in several ways. First, it had a relatively small patient population and had no comparison group and thus it is difficult to propose generalizable results. However, we believe that SFM and the dissection of the mesocolon in a cranial-to-caudal and medial-to-lateral fashion are safe procedures and can be standardized. Additionally, the study had a retrospective design, no standard protocol was performed for the evaluation of anastomotic leakage, and no data were available regarding postoperative follow-up of the patients.
Conclusions
Based on the results of the study, we consider that SFM has potential to reduce the intra- and post-operative complications associated with anastomotic leakage in laparoscopic resection of rectal cancer. Therefore, SFM performed via the superior-to-inferior and medial-to-lateral approach appears to be a safe and feasible procedure. The surgical procedure can be initiated by the division of the IMV in a superior-to-inferior and medial-to-lateral fashion. Further comparative prospective studies investigating the standardization of SFM and elimination of controversies related to this procedure are needed.