Purpose
Renal cell carcinoma (RCC) accounts for 3% of all cancer diagnoses and cancer-related deaths worldwide, and its incidence is growing [1]. The most common histologic sub-type is clear cell renal cell carcinoma, representing 70% of all RCCs [2]. Up to 20% of patients with newly diagnosed RCC present synchronous distant metastases, and 20% of patients with initially locoregional disease are expected to develop metastatic renal cell carcinoma (mRCC) during surveillance [3, 4]. The most common mRCC locations are lungs, bones, liver, and an opposite kidney [5]. Cutaneous metastases are occasional manifestations of mRCC, with the incidence varying from 2.8% to 3.8% [6, 7]. The development of molecular biology and genomics of RCC resulted in first- and second-line treatments of mRCC based on immunotherapy, including tyrosine kinase inhibitors (TKIs), targeted therapy against vascular growth factor (VEGF), inhibitors of mammalian kinase target of rapamycin (mTOR), and immune checkpoint inhibitors (ICI) of anti-programmed cell death-1 (PD-1)/programmed cell death ligand-1 (PD-L1) [8-10]. Median overall survival (OS) of patients with stage IV mRCC treated with a combination of ICI and TKIs or other targeted therapies varied from 26.0 to 35.7 months [11, 12]. According to the European Society for Medical Oncology (ESMO) guidelines, therapeutic options for patients who are not eligible for systemic therapy include observation, metastasectomy alone and external beam irradiation, with conventional radiotherapy (CRT), stereotactic radiosurgery (SRS), and stereotactic body radiation therapy (SBRT) [13, 14]. The present case report shows the potential benefits of high-dose-rate surface brachytherapy (HDR sBT) for cutaneous metastatic renal cell carcinoma (cmRCC).
Material and methods
Patient status
An 81-year-old female was admitted to our center with metastatic clear cell RCC. In 2004, the patient was diagnosed with a tumor located in the right kidney and underwent right nephrectomy. Histopathology report presented pT1a grade 1 (G1) adenocarcinoma clarocellulare tumor, with expression of CK AE1/AE3 (+), RCC (+), Pax8 (+), CD10 (+), VIM (+), and Ki67 50%. According to the ESMO guidelines for stage I RCC, no adjuvant treatment was administered, and the patient remained in surveillance [10]. In 2015, the patient was diagnosed with a 47 × 58 × 78 mm tumor mass located in the left kidney, and underwent intra-arterial transcatheter renal artery embolization. No material for histopathology was obtained, and the patient continued surveillance. In 2018, the patient manifested two painful on palpation abscesses in the left parietal and occipital region, which were initially treated by a dermatologist with topical steroids and fusidic acid. Following the lack of response to dermatological treatment, surgical excision was performed. Histopathology report confirmed metastatic spread of the clear cell RCC with positive surgical margins, and the patient was referred to medical oncology department. Due to an advanced age, WHO-III performance score, and multiple comorbidities, such as chronic heart failure NYHA III with pectoral angina CCS II controlled with implantable cardioverter-defibrillator (ICD), stage IV chronic kidney disease, insulin-dependent type 2 diabetes, and hypertension with three cerebrovascular accidents in the past, the patient was disqualified by a medical oncologist from palliative treatment, with TKIs or immunotherapy, and no further diagnostic imaging was performed. The patient was referred to brachytherapy department to consider local palliative treatment of painful lesions.
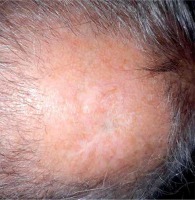
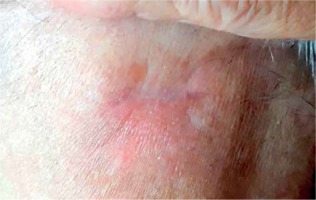
One month after the excision, on admission to our Center, the patient presented a scar with visible skin tumor relapse (1 cm in diameter) surrounded by an extensive palpable subcutaneous infiltration in left parietal region and a 2 cm long scar after the excision located in occipital region, with not visible but palpable subcutaneous residual mass. Both locations were painful to touch as well as at supine position, despite the use of oral tramadol 75 mg thrice daily. Additional two palpable but painless subcutaneous metastases located in right subscapular and left gluteal region were identified. A radiation oncologist qualified the patient for an attempt of HDR sBT for painful and function-comprising lesions, and further best supportive care (BSC).
Brachytherapy treatment
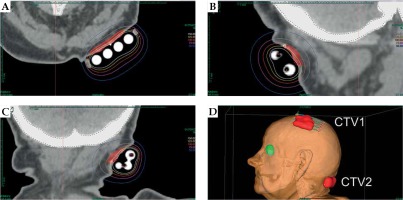
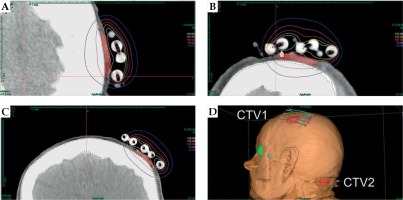
The patient was qualified for palliative HDR sBT of lesions located in the left parietal and left occipital regions. The tumors’ borders were set with on-skin markers (Figures 1 and 2), and two sets of individual flap applicators (Freiburg Flap, Elekta Brachytherapy) combined with five single-leader catheters for parietal and 2 for occipital tumor (Figure 3) were prepared and adjusted to the patient’s skin. Afterwards, the applicators’ positions were marked with a waterproof pen, and computed tomography (CT) scan of the head and neck region was performed with a 1 mm slice thickness. 3D treatment planning was completed with the OncentraBrachy system (Nucletron, an ELEKTA company, ELEKTA AB, Stockholm, Sweden) by delineating two clinical targets volumes (CTVs). CTV1 covered tumor in the left parietal region, with maximum depth of CTV = 4.5 mm and volume = 1.91 cc, while CTV2 included lesion in the left occipital region, with maximum depth of CTV = 3.5 mm and volume = 1.39 cc (Figures 4 and 5). There were no margins added to CTVs. Target volumes were specified basing on clinical examination, pathology report, and the lesion seen on CT scans. Organs at risk (OARs) were delineated, including adjacent bones and eye lenses. Subsequently, a medical physicist reconstructed the applicators digitally. The source positions above the CTVs were activated to plan an optimal dose distribution. The axis dose points were added and adapted to the thickness of the lesion, or the applicator points were added manually and adapted to the deeper surface of the lesion. Dose distribution was normalized to the defined points and then optimized using dose-point optimization with a distance option (Table 1). For CTV1, the physician and physicist decided to irradiate the target in the parietal region, using 4 out of 5 single-leader catheters due to sufficient CTV coverage and favorable dose distribution with 4 catheters. The total dose of 36 Gy (reference dose) in 6 fractions was planned, and two fractions per week were scheduled for both CTVs, closing the overall treatment time in 18 days. Outpatient treatment was performed simultaneously for both CTVs using a MicroSelectron HDR-BT unit (Nucletron, an Elekta Company, Stockholm, Sweden) equipped with an iridium-192 (192Ir) radioactive source, characterized by average radiation energy of 0.38 MeV and nominal activity around of 370 GBq (reference air-kerma rate, 41 mGy/h). Lead apron covering ICD location was applied at each fraction to ensure cardiac safety of the treatment.
Fig. 1
Tumor relapse after metastasectomy surrounded by extensive palpable subcutaneous infiltration in left parietal region. A set of on-skin markers were used to enable visualizing borders of target volumes on CT scan
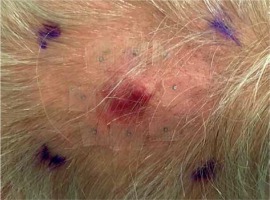
Fig. 2
Scar after metastasectomy located in left occipital region, with palpable residual mass. A set of on-skin markers were used to enable visualizing borders of target volumes on CT scan
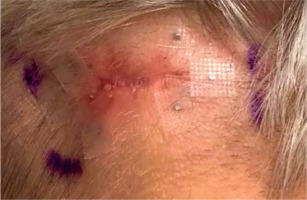
Fig. 3
Two sets of individual flap applicators (Freiburg Flap, Elekta Brachytherapy) combined with 5 single-leader catheters for parietal and 2 single-leader catheters for occipital tumor
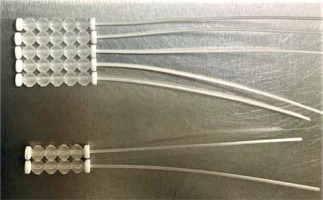
Fig. 4
Axial (A), sagittal (B), and coronal (C) CT-based reconstruction of CTV1 application localized in left parietal region with dose distribution. 3D reconstruction of contact applicators placed on both treated areas (D)
Results
The patient completed the scheduled treatment presenting grade 1 acute skin erythema, according to Radiation Therapy Oncology Group/European Organization for Research and Treatment of Cancer (RTOG/EORTC) Radiation Toxicity scoring. Two weeks after HDR sBT, the tumor in the parietal region (Figure 6) presented partial clinical response and complete clinical response, with no palpable tumor observed in the occipital region (Figure 7). Adjacent skin demonstrated local healing process, with RTOG/EORTC grade 1 erythema with dry desquamation, alopecia, and pruritus. Similar clinical status with proper healing process was observed 6 weeks after the treatment, with a significant additional reduction of oral analgesics (reduction of morphine equivalent from 25 to 15 mg). Three and five months after the procedure, the patient presented complete clinical response in the parietal region (Figure 8) and no clinical signs of relapse in the occipital region (Figure 9), with a further reduction of opioid drugs usage. Both irradiated regions presented RTOG/EORTC grade 1 skin depigmentation and grade 2 hair loss. However, clinical progression of non-irradiated cutaneous metastases was noted, with one additional subcutaneous tumor in the right forearm. The patient died 7 months after the completion of HDR sBT due to sudden cardiac death, with no clinical signs of progression in the irradiated fields.
Fig. 6
Parietal region two weeks after surface brachytherapy showing partial clinical response. RTOG/EORTC grade 1 erythema with dry squamation and alopecia visible
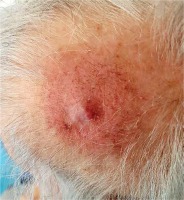
Fig. 7
Occipital region two weeks after surface brachytherapy showing complete clinical response with no palpable residual mass. RTOG/EORTC grade 1 erythema with dry squamation and alopecia visible
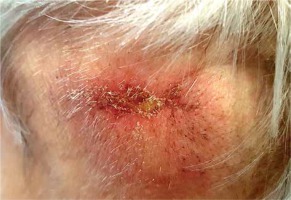
Discussion
Cutaneous metastases of RCC are rare manifestations of mRCC, and represent 6% of all cutaneous metastases [15]. Median OS of patients with cmRCC varies from 6 to 12 months, and is worse compared to 26-36 months in patients with non-cutaneous metastases [16, 17]. Treatment modalities, which can improve OS for patients with mRCC, include systemic therapy alone or combined with cytoreductive nephrectomy, stereotactic ablative radiation therapy (SABR), and metastasectomy. However, considering the potential drug resistance and significant toxicity associated with these modalities, an alternative approach may be required [13, 14, 18, 19]. The role of radiation therapy in the treatment of mRCC was limited to symptomatic palliative radiation therapy due to the common opinion that RCC is relatively radioresistant comparing to other tumors [20]. The opinion was based on multiple studies on in vitro RCC cell lines. Syljuasen et al. and Ning et al. demonstrated that the surviving fraction of RCC cell lines after irradiating with a single dose of 2 Gy (SF2) ranged from 0.37 to 0.60, presenting the highest rate from all examined cell types [21, 22]. Leung et al. presented that RCC cell lines vary in radiosensitivity depending on glutathione concentration, explaining unexpected variation in SF2 [23].
However, Deschavanne et al. in their meta-analysis indicated that α/β ratio (ratio describing tissue sensitivity to change of fraction dose or dose-rate) used to present biologically effective dose (BED) in linear-quadratic model is relatively low (between 2.6 and 4.2), highlighting that potential benefit can be obtained using higher than conventional fractionation dose [24]. The tendency to use hypofractionation in RCC oligometastatic radiotherapy is currently expressed in the number of ongoing clinical trials investigating the use of SABR with encouraging LC and OS results, but these reports relate to visceral metastatic disease only [18, 25-27]. Gay et al. presented the only accessible in the literature case report on the treatment for cutaneous mRCC with hypofractionated EBRT [28]. The five-week-long treatment using electron beam radiotherapy and a custom on-skin bolus, included 13 fractions of 3.75 Gy to a total dose of 48.75 Gy for a 2 cm tumor located in a preauricular region. It resulted in complete clinical response with a good normal tissue tolerance and significant pain reduction, until unrelated to irradiated field death of patient 10 months after the treatment. To describe this regimen’s radiobiological features, the authors used α/β ratio equal to 10 for tumor response, which seems to be overstated regarding the above-mentioned scientific reports. A different radiobiological foundation is shown in the present case study by using a three-week-long regimen with 6 Gy per fraction, twice weekly to a total dose of 36 Gy, using HDR sBT for definite treatment. The total delivered dose and fractionation schedules aimed at pain alleviation, function restoration, complete local eradication of cancer cells, and shortening of overall treatment time (OTT). This regimen’s rationale was based on the assumption that tumors with low α/β ratio, such as cmRCC, are more prone to fractionation dose variability than skin tumors with high α/β ratio, i.e., squamous cell skin carcinomas [29]. Therefore, the total physical dose de-escalation, reduction of the normal tissues irradiated volume, and shortening of OTT were allowed. The efficiency of both regimens were evaluated using BED and equivalent doses in 2 Gy fraction (EQD2). For the purpose of this comparison, α/β ratio = 2.6 was adapted, and BED was calculated using equation BED = n × d (1 + d/α/β), where n is the number of treatment fractions, d – dose per fraction in Gray (Gy), and α/β – α/β ratio [30]. BED2.6 for sBT regimen was 119.1 Gy and for hypofractionated EBRT, BED2.6 was also 119.1 Gy. EQD2 were also counted for both scenarios using equation formula EQD2 = D × ([d + (α/β)]/[2 + (α/β)]), where D is total dose given in Gy, d is dose per fraction in Gy, and α/β is α/β ratio, resulting in EQD2 = 67.3 Gy for sBT and hypofractionated EBRT regimens. Both schedules provided the same treatment results and normal tissue toxicity, while the sBT regimen allowed the delivery of total prescribed doses in a more convenient pattern, with fewer fraction-related hospital visits and shorter OTT than the EBRT regimen. Furthermore, sBT allowed to omit dose build-up at the skin surface; thus, it did not require on-skin bolus, enabling more heterogenic dose distribution in more challenging locations, as compared to EBRT [31]. Late normal tissue tolerance and cosmesis assessment require longer observation period, which may be difficult considering poor OS in this group of patients; nonetheless, the post-treatment cosmesis after sBT is expected to improve, as compared to EBRT [32]. Zaorsky et al. in SCRiBE meta-analysis evaluated data of 24 studies focusing on clinician-reported post-treatment cosmesis of 3,399 patients with basal cell carcinoma or squamous cell carcinoma, treated with either EBRT or sBT. To compare BEDs of applied fractionation regimens, α/β ratio of 3.0 was adapted, and BED3Gy were calculated. For regimens with BED3Gy > 100 Gy (which included both EBRT regimen used by Gay et al. [28] and sBT regimen presented in our case study), post-treatment cosmesis was favorable for sBT procedures when compared to EBRT regimens: 95% of sBT treated cases were classified as “good” cosmesis, while 79% of EBRT treated cases were classified as “good” with an increasing amount of “fair” cosmesis. Further investigation with a larger group of patients is needed to assess long-term local control, feasibility in both palliative and oligometastatic scenarios, and possible profits arising from sequential use of immunotherapy with sBT, including abscopal effect remaining metastatic lesions [33].
Conclusions
High-dose-rate sBT can be a useful definite treatment option for focal renal cancer skin metastases, especially for patients who are not eligible for other treatment modalities or lesion causing organ dysfunction and/or pain. The flap applicators and 3D treatment planning system allowed to optimize the dose inside CTVs while sparing normal tissues. The provided treatment resulted in excellent local control and reduced pain level. To the best of our knowledge, the presented case study is the first to report the feasibility and efficiency of sBT in management of cmRCC.