INTRODUCTION
Stroke is one of the leading causes of disability and the second leading cause of mortality worldwide. It is estimated that one in four individuals will experience a stroke, with approximately 15 million new cases occurring annually1,2. In Colombia, stroke ranks among the top five causes of mortality, with a reported rate of 32 deaths per 100,000 inhabitants3. In the city of Cali, approximately 3,101 stroke cases are registered each year, of which around 2,636 are ischemic strokes4.
Stroke is a neurological disorder characterized by the obstruction of cerebral blood vessels due to a blood clot, leading to reduced cerebral blood flow and subsequent brain damage5. The clinical manifestations of stroke vary depending on the location and severity of the lesion, with hemiplegia being one of the most common motor sequelae6.
Hemiplegia is characterized by muscle paralysis on one side of the body and may be accompanied by other symptoms, such as sensory, cognitive, and language impairments7. One of the most common sequelae is motor involvement, resulting in functional limitations that affect gait pattern. These gait alterations hinder mobility and performance in activities of daily living8,9. The rehabilitation of hemiplegia aims to restore motor function and improve independence through various therapeutic strategies. Among these, transcutaneous electrical stimulation (TENS)10 has shown promising effects in reducing spasticity, enhancing muscle strength, and managing pain associated with musculoskeletal disorders11.
TENS is a peripheral electrical stimulation technique that delivers low- and high-frequency (50–150 Hz) electrical currents through the skin12. Although traditionally used for pain relief13, studies suggest that TENS may also influence muscle strength and spasticity in patients with neurological conditions14,15. These effects are believed to result from the activation of afferent fibers of different diameters16, hemodynamic changes during electrically-induced muscle contractions17, reduced stretch reflex excitability, and presynaptic inhibition18.
TENS is a noninvasive, cost-effective therapy that can be applied using portable devices; as such, it is an accessible option for both clinical and home-based rehabilitation19. However, while existing studies indicate that TENS can improve muscle strength, reduce spasticity, and enhance motor function in hemiplegic patients, its optimal dosage, duration, and effects on gait remain unclear. Despite the existence of previous literature reviews on the use of TENS in post-stroke patients, critical gaps remain that necessitate further investigation. Many prior reviews have primarily examined the effects of TENS on motor recovery, pain management, or spasticity reduction, often without a specific emphasis on functional mobility and gait rehabilitation. Additionally, inconsistencies in methodology and stimulation parameters, and a lack of standardized outcome measures in previous reviews limit the ability to draw definitive conclusions about the efficacy of TENS in improving gait. Given these uncertainties, this systematic review aims to analyze the effects of TENS on gait and daily function in stroke survivors, providing insights into its potential role in post-stroke rehabilitation.
MATERIALS AND METHODS
This study was conducted following the Cochrane Collaboration recommendations and the PRISMA statement. The protocol was registered in the International Prospective Register of Systematic Reviews (PROSPERO), under number CRD42023488243.
Eligibility criteria
This review included experimental, quasi-experimental, cohort, case-control, and cross-sectional studies. All involved patients with hemiplegia, and evaluated TENS application protocols and gait performance through various parameters, including speed, distance, cadence, step amplitude, and specific gait analyzers. The secondary outcome examined activities of daily living. The exclusion criteria encompassed studies involving children and adolescents, patients with cranioencephalic trauma or neurodegenerative diseases, electrotherapy protocols using currents other than TENS, studies that did not assess gait as a primary outcome, narrative reviews, case studies, letters to the editor, and expert opinions, studies with insufficient methodological quality or incomplete data reporting and animal studies or research conducted on in vitro models.
Data sources
The following databases were used: MEDLINE (OVID), ScienceDirect, LILACS, WEB OF SCIENCE, and the Cochrane Central Register of Controlled Trials (CENTRAL). Controlled clinical trials, case-control, cohort, and cross-sectional studies were included from inception to November 2023. To ensure full coverage of the literature, the search also included relevant papers identified through the references, as well as congresses, thesis databases, Open Grey, Google Scholar, and Clinicaltrials.gov. The search was performed using DeCS/Mesh terms and related words with different combinations of Boolean operators. No language or time restrictions were imposed.
Data collection
Data were extracted by two investigators. Both reviewed each paper based on the title and abstract. Following this, the full texts of the relevant studies were read, based on the inclusion and exclusion criteria mentioned above, and further data were obtained. Disagreements were resolved by consensus; when there was no agreement, a third reviewer participated.
Using a standardized form, two trained reviewers independently extracted the following information from each article: study design, location, authors’ names, title, purposes, number of patients included, time, outcome definitions, results, and association measures.
Risk of bias (quality) assessment
The quality and risk of bias of nonrandomized studies were assessed using the methodological index for nonrandomized studies (MINORS) score20 in an independent and blinded manner, including studies with a score higher than 11. Clinical trials, were assessed using the PEDro scale21, which evaluates randomization and blinding, and result processing. Studies with a score of 6–10 were considered to have high methodological quality, and these were included. Discrepancies between authors during the quality assessment were resolved by discussion; if no agreement was reached, a third author was consulted.
RESULTS
Study Selection and Eligibility Criteria
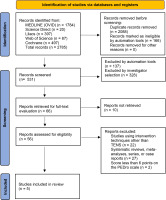
A total of 2 785 studies were initially identified through the database search. After removing duplicates, 531 studies remained for title and abstract screening. At this stage, 465 studies were excluded for being systematic reviews, letters to the editor, intervention protocols, or unrelated to the topic of interest.
The five22,26 controlled clinical trials met all eligibility criteria and were included in this review (Figure 1). The included studies were conducted in Asia, with two from Hong Kong22,23, two from Korea24,26, one from Seoul25. All were published in English (Table 1).
Table 1
Characteristics of the included studies
Methodological quality
As shown in Table S1, the mean PEDro scale score for the included studies was 8. The most frequent omissions were observed in concealed allocation and blinding of therapists when administering therapy.
Characteristics of participants
Table 2 presents the characteristics of participants. Age ranged from 52 to 71 years, all studies included populations of both sexes22,26 and included samples from 2925 to 109 participants23. The side affected by hemiplegia was reported in two studies22,23, with the left hemisphere being the most frequent. Poststroke time was given in months and years, and specified in three studies22,25,26, ranging from one month to six years.
Table 2
Characteristics of the Intervention
TENS Application Protocols
All studies used the same application protocol for TENS 22,26, with parameters set at 100 Hz and 200 μs pulse width, applied at twice the sensory threshold intensity. Treatment duration varied, with three studies24,26 using 30-minute sessions and two studies22,23 applying 60-minute sessions. Electrode placement also varied among studies: two studies24,26 targeted the common peroneal nerve pathway, two 22,23 placed electrodes on Chinese acupuncture points (ST 36, LV 3, GB 34, UB 60), and one25 positioned electrodes over the muscle belly of the quadriceps and gastrocnemius (medial and lateral areas) (Table 2).
Effects on Gait Parameters
Lower Limb Spasticity: Four studies22,24,26 assessed spasticity reduction, with all reporting significant improvements in gait parameters. All studies used a five-day-per-week intervention schedule with an average duration of six weeks. Three of these studies implemented 30-minute TENS sessions targeting the common peroneal nerve pathway24,26 and one study used 60-minute sessions with Chinese acupuncture point placements22. All studies demonstrated a significant reduction in spasticity and improvements in gait speed and muscle strength (dorsiflexors and plantar flexors) in patients receiving TENS compared to placebo groups (Table 3).
Table 3
Results of protocol interventions in spasticity
Gait Speed: Five studies22,26 evaluated gait speed, with four22,25 combining TENS with task-related exercises. These studies reported significant improvements in gait speed for patients receiving TENS + exercise compared to those receiving TENS alone or placebo, with the TENS + exercise group sustaining long-term improvements (Table 4).
Table 4
Results of protocol interventions in walking speed (cm/sec)
Muscle Strength and Functional Mobility: Three studies22,24,26 assessed plantar flexor and dorsiflexor muscle strength using a load cell on a standing frame22 and a handheld dynamometer24,26. Two studies24,26 applied 30-minute TENS sessions targeting the common peroneal nerve pathway. One22 used a 60-minute protocol with acupuncture-based electrode placement. Significant improvements in muscle strength were observed in patients receiving TENS + exercise/task-based interventions compared to those receiving TENS alone or placebo (Table 5).
Table 5
Results of protocol interventions in muscle strength
Other Gait-Related Variables and Functional Performance: Beyond gait speed and muscle strength, other variables such as balance, endurance, and functional mobility were assessed (Table 6).
Table 6
Results of protocol interventions in other variables
Balance: One study25 used the Good Balance device and timed up-and-go test, showing significant improvements in static and dynamic balance when TENS was applied to the quadriceps and gastrocnemius.
Endurance and Functional Gait Mobility: One study23 used the 6-minute walk test, finding greater improvement in TENS + exercise groups compared to the control group, with sustained effects over time. Additionally, the study found significant improvements in TENS-treated patients, but greater and longer-lasting benefits were observed in those who received TENS + exercise interventions.
DISCUSSION
The findings of our systematic review show that the combination of TENS with therapeutic exercises positively affects gait and performance in daily living in patients with hemiplegia secondary to stroke; it appears to do so by relieving spasticity and improving inter alia speed, functionality, and gait endurance.
Application of TENS for four to six weeks, five times a week, decreases spasticity in patients with hemiplegia; should be applied as a 100 Hz current with a pulse width of 200 μ, at twice the sensory threshold, over the common peroneal nerve pathway.
In patients with hemiplegia, common peroneal nerve dysfunction is commonly associated with gait abnormalities resulting in foot drop27; electrical stimulation has been shown to improve ankle dorsiflexion, balance, and functional mobility in this population28, even in functional task-related training. In 2019, Mahmood et al.29 reported that TENS reduced limb spasticity when administered in combination with other forms of physical therapy for more than 30 minutes. This is consistent with the results reported by Laddha et al.30, who concluded that the use of TENS for 30 or 60 minutes can effectively reduce spasticity in the ankle plantar flexors, thereby increasing gait ability and enhancing the effectiveness of task-related training. Shorter application times do not seem to be effective for treating spasticity: Picelli et al.31 do not note any significant differences between 15-minute TENS application to the tibial nerve and the use of ultrasound on the gastrocnemius in patients with spastic clubfoot.
Decreased strength in the periarticular muscles of the ankle contributes to alterations in gait pattern, and reduced speed and alterations in the stance and swing phases, which are controlled by the plantar and dorsiflexor. The studies included in this review reported that the combination of task-related exercises and the application of TENS to the common peroneal nerve motor point significantly improved muscle strength of the dorsiflexors and plantar flexors compared to the use of TENS alone. This finding is consistent with those of Yan et al.32, who reported that the use of TENS administered five times a week within 10 days after stroke significantly reduced plantar flexor spasticity and increased ankle dorsiflexor strength.
Moreover, these parameters were effective for other gait-related variables, such as gait speed and gait endurance, when current was applied to the hemiplegic side. However, a greater long-term effect was observed in protocols in which the current was applied together with task-related exercises. In these patients, the use of TENS appears to be particularly useful as adjunctive therapy in a task-related training program for the improvement of motor skills33.
In addition to strength and gait speed, an essential component for walking is balance. Kyoung-Sim Jung et al.34 proposed that the use of TENS significantly helps the dynamic balance of patients with stroke sequelae when performing functional movements such as standing up and sitting down from a chair. This is consistent with the results obtained in one of the analyses considered in this review, where a significant improvement in the ability to stand up without loss of balance was reported following four-week application of TENS current to acupuncture points of the paretic leg.
The use of therapeutic currents to improve performance and independence in activities of daily living has also been reported in other studies, such as that of Liu, et al.35, where they demonstrated that the use of TENS for 30 min with frequencies of 20 Hz had positive effects in poststroke patients, and improved urinary incontinence and performance in activities of daily living assessed with the Barthel index. In this review, a significant improvement above the mean Barthel index score was observed, especially in the items of bladder control, feeding, transfers, and mobility.
Strengths and limitations
This review highlights the therapeutic potential of TENS in improving gait and daily function, and provides valuable insights regarding evidence-based clinical practice and rehabilitation strategies for stroke survivors. Unlike previous reviews that primarily focused on motor recovery or pain management, this study emphasizes the functional effects of TENS in this population. It also employs a rigorous methodology, including a structured search strategy, clearly-defined inclusion and exclusion criteria, and quality assessment using the PEDro scale, ensuring the reliability of the selected studies.
However, this review is potentially limited by the small number of studies evaluating the effects of TENS on gait parameters. Additionally, the variability in outcome measures used to assess gait and daily function makes it challenging to draw definitive conclusions and establish standardized recommendations for clinical practice.
Implications for Further Research
Future research should focus on standardizing TENS application protocols by defining optimal stimulation parameters, treatment duration, and electrode placements to enhance its clinical effectiveness. Additionally, larger, high-quality randomized controlled trials with long-term follow-up are needed to assess the sustained effects of TENS on gait and daily function in stroke survivors.
CONCLUSIONS
The use of TENS in the treatment of patients with stroke sequelae is a useful strategy to optimize activities of daily living and gait. However, a careful and detailed evaluation of each patient is required, taking into account contextual factors, individual conditions, and clinical characteristics.
The analysis of the results showed a positive improvement in variables related to gait and activities of daily living. Nevertheless, despite the positive results, some of the limitations of this review are related to the variability in the application times of the protocols and the variability in sample sizes and characteristics of the participants included. Therefore, further research should be conducted to provide more understanding of the long-term effects of TENS and to establish an effective application protocol for rehabilitation programs.