Purpose
Intermediate-risk (IR) group for prostate carcinoma (PCa) is probably the group that includes the most heterogeneous patients in this wide pathology. Radical prostatectomy, external beam radiotherapy (EBRT), or brachytherapy (BT) exclusively or in combination with or without androgen deprivation therapy (ADT), are the treatments recommended for this group of patients [1].
Dose escalation in PCa has demonstrated higher levels of prostate specific antigen (PSA) control rates [2-6], in specific trials with IR patients [7, 8].
In this manuscript, we present mono-institutional retrospective results from completed database of PCa patients diagnosed with IR, and then treated, in all cases, by the same team of professionals, using a combined treatment of EBRT and BT [low-dose-rate (LDR) 125I seeds permanent implants or high-dose-rate (HDR)].
To the best of our knowledge, this is the first comparative study dealing exclusively with IR PCa comparing LDR-BT and HDR-BT as a boost in the same institution. The main characteristics of this retrospective cohort was the uniformity of therapeutic decisions, treatment performed, and follow-up. All treatments were carried out by the same radiation oncologist. Moreover, the team of physicists followed a strict protocol to assure uniformity, which guaranteed that EBRT and BT contouring and technical process maintain a high degree of homogeneity.
The primary endpoint of this study was to compare the boost outcomes of two BT techniques, LDR and HDR, in terms of biochemical progression-free survival (bPFS) and toxicity. We have re-classified patients into “favorable” and “unfavorable” IR sub-groups (FIR and UIR) in order to identify potential result differences [9]. The secondary endpoint was to analyze any differences in both prognosis sub-groups that received treatments of similar intensities.
Material and methods
Patient characteristics
From January 2005 to February 2018, 188 patients diagnosed with IR PCa were treated with EBRT and BT in our department. Forty-six patients had no record of a post-BT PSA from a follow-up (since they were referred from other centers), and were therefore excluded from this study analysis (34 treated with LDR and 12 treated with HDR), leaving 142 patients eligible for inclusion. From January 2005 to June 2012, BT boost in IR patients was applied with LDR-BT, changing to HDR-BT boost when this prostate BT technique was incorporated in the department in July 2012. The Radiotherapy Department at our hospital is a reference center for patients from other clinics. Therapeutic decision is undertaken by various committees dealing with urological tumors in referring centers. Since 2005, the accepted protocol is a combined treatment of EBRT + BT + 6 months of ADT in patients diagnosed with IR PCa, according to the guidelines [1]. The cases treated exclusively with EBRT are based on personal decisions of patients or BT procedure contraindications. Between January 2005 and February 2018, 142 patients with a proven diagnosis of histopathologic IR PCa (D’Amico classification) [10] were treated with a BT boost with a complete follow-up (June 2020). The outcomes were then retrospectively analyzed.
All patients were included in IR based on baseline PSA (10-20 ng/ml), Gleason ≤ 7, or stage T2b-T2c [evaluated with transrectal ultrasound and/or pelvic magnetic resonance imaging (MRI) and computed tomography (CT) scan of the abdomen and pelvis]. Patients were re-classified into FIR and UIR sub-groups. UIR was defined as the presence of 2 or 3 IR risk factors and/or Gleason 7 (4 + 3) (ISUP, grade 3), and/or ≥ 50% of positive core biopsies.
EBRT characteristics
All patients were treated by EBRT with intensity-modulated radiotherapy therapy (IMRT), using seven statics fields with sliding-window technique or volumetric modulated arc therapy (VMAT), when the latter became available in our institution (December 2013). In both cases, the conformity level and organs at risk (OARs) sparing were equivalent, VMAT was adopted due to its higher treatment delivery efficiency. Linac and treatment planning system were performed using a Clinac 2100 CD and Eclipse, respectively (Varian, Palo Alto, USA). Clinical target volume (CTV) was the prostate and seminal vesicles. An additional margin (10 mm anterior, posterior, and lateral, and 8 mm superior and inferior) was added for planning target volume (PTV). The prescribed dose was 45 Gy (1.8 Gy/fraction) using boost with LDR-BT and 60 Gy (2 Gy/fraction) with HDR-BT. A dose of 45 Gy with EBRT was administered in case of a boost with LDR-BT, as recommended by the ABS [11]. When the HDR-BT boost program commenced in our institution, the literature was first reviewed, and it was decided to select 60 Gy in 30 fractions [12-14]. EBRT was delivered in all patients before the BT boost.
Brachytherapy characteristics
Brachytherapy treatment was performed in all the patients using spinal/epidural anesthesia and sedation with an intra-operative procedure by a transperineally approach.
From January 2005 to June 2012, BT boost in IR patients was performed employing LDR-BT (permanent 125I seeds). The planning and seed delivery were performed with a transrectal ultrasound-guided (TRUS) unit (BK Medical Falcon 2101 EXL) in real-time using Fully Integrated Real time Seed Treatment (FIRST) system (Nucletron, Elekta AB, Stockholm, Sweden). The FIRST system consists of a seed Selectron-associated hardware and two software environments with an endocavity rotational mover (ECRM) attached to the needle stepper to acquire and reconstruct 3D volumetric data by rotating a bimodal ultrasound. The seed Selectron incorporates a diode array to verify a build sequence and assay seed source strength. Finally, this system utilizes a remote afterloader to deliver LDR 125I seeds into the patient. The system provides an intra-operative interactive planning, virtual needle guidance, robotic seed delivery (loosed seeds), and needle retraction system. SPOT PRO (Nucletron, Elekta, Stockholm, Sweden) system was used for treatment planning.
Prostate gland (declared as CTV), urethra, and rectum were contoured. Dose was prescribed for the prostate, with a 2 mm isometric margin (except posteriorly, where no margin was applied) following ESTRO/EAU/EORTC recommendations [15, 16]. The aim was to achieve a D90 equal or higher to the 100% of prescribed dose, with V150% ≤ 50% and D2cc ≤ 100% for the rectum. The prescribed dose was 100 Gy in 13 patients (26%) and 108 Gy in the remaining 37 cases (74%). The dose limit given to the urethra was constrained to 135% of the prescribed dose, based on a previous radiobiological study using a linear-quadratic model [17]. A post-plan was constructed for all patients one month after the implant, fusing an axial CT scan study (CVT ASIR, General Electric, Wisconsin, USA) and an MRI T2 axial sequence (1.5T MRI, General Electric, Wisconsin, USA).
In July 2013, we commenced to employ a boost with real-time HDR-BT (192Ir). Treatment plans were created and optimized using a treatment planning system Oncentra Prostate (Elekta, Stockholm, Sweden). HDR boost was performed under real-time TRUS guidance. Plastic needles were inserted transperineally into the prostate. TRUS-based planning objectives included the prostate plus a 2 mm of margin (except posteriorly), with a D90 ≥ prescription dose, i.e., > 100%, following GEC/ESTRO recommendations [18], keeping the urethra dose below 120% of the prescribed dose. Dose prescription was 10 Gy in 1 fraction brachytherapy procedure, which was executed on a MicroSelectron v 2 remote afterloader treatment system (Elekta, Stockholm, Sweden).
LDR-BT: Median number of seeds 55 (26-100). Median air kerma strength per seed 0.432 U (0.305-0.660 U), being 1 U = 1 μGym2h–1. Median intra-operative planning D90 was 115.25 Gy (100.3-135 Gy). The 125I source model used was a SelectSeed from Elekta, and calculations were based on TG-43 using AAPM consensus datasets.
HDR-BT: Median prostate D90 was 10.75 Gy (10.05-11.7 Gy). This value was slightly higher than the prescription dose, prioritizing a coverage greater than 90%. The 192Ir source model was a MicroSelectron HDR v2 from Elekta, and calculations were based on TG-43 using AAPM-ESTRO consensus datasets.
The same radiation oncologist, who performed EBRT treatments, completed brachytherapy procedures, unifying the technical and dosimetric criteria.
Follow-up
After the treatment, 100% of the patients had a personal follow-up by the same radiation oncologist. According to our departmental protocol, this involved patients having a 4 monthly PSA for the first year, and then every 6 months until fifth year, and then annually. PSA elevations of nadir + 0.4 during follow-up and a normalization without any treatment was consider a bouncing phenomenon. Biochemical failure (BF) was defined as change of nadir + 2 (Phoenix definition). When BF was evidenced, chest and abdomen CT, pelvic MRI, bone scan, or choline positron emission tomography (PET-CT) was performed.
Toxicity
For toxicity analyses, the common terminology criteria for adverse events (CTCAE) version 4.0 was employed [19], examining acute toxicity in the first 3 months after the BT treatment. Toxicity was reported in every patient, with the same frequency described in the follow-up chart, which was retrospectively recoded.
Statistical methods
IBM SPSS Statistics version 15.0 (IBM, USA) was used for statistical analysis. Descriptive statistics were applied to compare prognostic factors. Baseline characteristics were compared using t-tests for continuous variables, assuming normality of the samples, and chi-square tests for categorical variables. Categorical variables were listed as frequencies and percentages, and continuous variables were presented as medians (interquartile range). Kaplan-Meier method with log-rank test was used to calculate the actuarial bPFS, overall survival (OS), cause-specific survival (CSS), local failure (LF), lymph node failure (LNF), distant failure (DF), and comparisons. bPFS rate was calculated for all living patients. Cox proportional hazards regression model was utilized for univariable (UVA) and multivariable analysis (MVA) to assess factors associated with biochemical relapse-free survival. For the bPFS, OS, CSS, LF, LNF, and DF endpoints, UVA included age, percent positive cores (PPC), Gleason score (≤ 6 vs. 7), FIR vs. UIR, and the type of BT boost technique (LDR or HDR). We analyzed risk factors for toxicity comparing both techniques of BT boost. Cox proportional hazards analysis was performed to calculate odds ratios (ORs) and confidence intervals (CIs), evaluating the influence of patient’s tumor and treatment characteristics on the risk of toxicity. MVA was performed using a logistic regression model. A p value of ≤ 0.05 was considered statistically significant.
Overall follow-up was calculated as the length of time taken to finish the combined radiotherapy treatment to the last contact follow-up.
Results
The median age of total cohort at diagnosis was 70 years (range, 51-81 years). Twelve patients (9%) were staged as T1c, 16 (11%) T2a, 17 (12%) T2b, and 97 (68%) T2c. The median Gleason score was 6 (range, 2-7). Gleason score was ≤ 6 in 64 patients (45%), Gleason score was 7 in 63 patients (44%), and in 15 patients (11%), it was defined as well- or moderate-differentiated carcinoma. Median PSA was 9.2 ng/ml (range, 3.37-20 ng/ml). A total of 141 (99%) patients received ADT for a median of 6 months (range, 6-24 months). Fifty patients were treated with LDR-BT (35%) and 92 with HDR-BT (65%). Clinical basal characteristics of both BT boost groups are described in Table 1.
Table 1
Clinical characteristics at diagnostic of both BT groups (LDR and HDR)
Forty-two patients (30%) were re-classified in FIR and 100 (70%) in UIR. Of the last group, 86 patients (86%) were due to 2-3 risk factors, 25 patients (25%) with biopsies of Gleason 7 (4 + 3), and 41 of 85 patients were the result of ≥ 50% positives biopsy cores. Twenty-two patients of the FIR group were treated with LDR-BT (52%) and 20 with HDR-BT (48%). Twenty-eight patients of the UIR group were treated with LDR-BT (28%) and 72 with HDR-BT (72%). Treatment characteristics of both BT techniques have been previously described.
The median overall follow-up (June 2020) for the entire cohort was 66.5 months (range, 16-185 months). The median follow-up was 117.5 months (range, 34-185 months) and 54 months (range, 16-90 months) for the LDR-BT and HDR-BT boost, respectively.
The median PSA at the last follow-up was 0.01 ng/ml (range, 0-132 ng/ml) and 0.06 ng/ml (range, 0-65 ng/ml) for the LDR-BT and HDR-BT patients, respectively. Four patients (8%) of the LDR-BT boost and 13 patients (14%) of the HDR-BT boost experience a bouncing in a median time of 21.5 months (range, 18-24 months) and 12 months (range, 6-28 months), respectively. The median value of PSA elevation was 1.23 ng/ml (range, 0.90-1.97 ng/ml) and 1.03 ng/ml (range, 0.42-2.6 ng/ml) in LDR-BT and HDR-BT groups, respectively.
bPFS was 93% and 91% at 60 and 90 months, respectively, in the total series. At 60 and 90 months, bPFS was 94% and 92% for LDR-BT boost and 92% and 92% for HDR-BT boost, respectively (log-rank test, p = 0.615). Figure 1 illustrates bPFS for LDR-BT and HDR-BT boost patients. The Kaplan-Meier OS for the total series was 93% and 84% at 60 and 90 months, respectively. The CSS were 98% at 60 months and 98% at 90 months. Ten patients (7%) experienced BF, 4 of them were treated with LDR-BT and the remaining 6 with HDR-BT. There was only one local failure in a patient treated with LDR-BT boost at 92 months after the treatment. This patient was salvaged with a second BT treatment with HDR (3 fractions of 10 Gy and 2 years of ADT). The patient is alive, with a biochemical control at 50 months after the salvage treatment. Five patients (4%) developed lymph node failure, one treated with LDR-BT and the others 4 with HDR-BT. Five patients (3%) developed metastatic disease, 2 patients in the LDR-BT group and 3 in the HDR-BT group. One patient treated with LDR-BT boost died with BF without an evidence of local or distant disease. In total, 20 non-prostate cancer deaths (14%) were recorded during the follow-up period.
Fig. 1
Kaplan-Meier plot showing proportions of patients treated with LDR-BT boost (blue curve) and HDR-BT boost (green curve), remaining free of biochemical progression
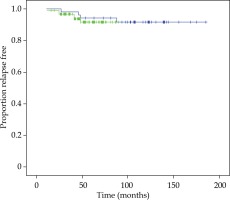
There was no significant difference in bPFS, OS, CSS, LF, LNF, and DF between both techniques of BT boost. bPFS at 90 months was 100% for FIR patients and 89% and 85% for UIR at 60 months and 90 months, respectively (log-rank test, p = 0.017). All patients with BF were included in the UIR group, 90% of them with 2 or 3 risk factors and 90% with PSA ≥ 10 ng/ml. Figure 2 demonstrates bPFS for FIR and UIR patients. There was not significant difference in OS, CSS, local control, lymph node failure or distant failure between patients with FIR or UIR and in this last group between the techniques employed for the boost (Table 2).
Fig. 2
Kaplan-Meier plot showing proportions of patients of favorable intermediate-risk (blue curve) and unfavorable intermediate-risk (green curve), remaining free of biochemical progression
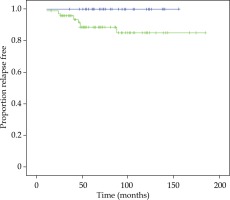
Table 2
Clinical results of favorable intermediate- risk (FIR) and unfavorable intermediate-risk (UIR) patients
Acute and chronic toxicity data are listed in Tables 3 and 4. The highest acute and chronic genitourinary (GU) and gastrointestinal (GI) toxicities were recorded at each visit and graded from 0 to 5 according to CTCAE. The crude incidence of acute grade 3 GU toxicity was 0.7% (1 patient) in the LDR-BT boost group. There was no acute grade 3 GU toxicity when HDR-BT boost was employed. The crude incidence of chronic grade 3 GU toxicity was 4% (2 LDR/4 HDR). One patient (0.7%) treated with HDR-BT had an acute urinary retention needing a catheter for 10 days. Five patients (3.5%) suffered from chronic urinary retention, which was solved with a transitory catheter; 3 of them were treated with LDR-BT boost and 2 with HDR-BT. Five patients (3.5%), (2 LDR and 3 HDR) presented with urethral stricture grade 3; 1 patient was treated with transurethral resection of prostate (TURP) and the other four with endoscopic urethrotomy. There was only one patient (0.7%) treated with HDR who had chronic hematuria grade 3. Chronic grade 1 rectal bleeding was superior (p = 0.03) in patients treated with HDR-BT. Twelve patients (8%) needed cauterization with argon (grade 2) (4 LDR and 8 HDR) without statistical significance between the boost technique employed. There was no chronic grade 3 GI toxicity. No GU or GI acute or chronic grade 4-5 toxicity was observed.
Table 3
Acute toxicity (CTCAE v4) BT boost
Table 4
Chronic toxicity (CTCAE v4) BT boost
Discussion
The 2017 American Society of Clinical Oncology (ASCO)/Cancer Care Ontario (CCO) brachytherapy guidelines state that brachytherapy boost (either LDR or HDR) should be offered to eligible IR PCa patients [20], based on three randomized trials [21-23]. These demonstrate improved disease-free survival in patients treated with brachytherapy combined with external beam, compared to those treated with external beam exclusively.
Both techniques demonstrated their effectiveness, with certain advantages of HDR over LDR, including greater consistency of dose distribution, radiobiological effect (large dose per fraction), real-time planning, absence of seed migration, radiation protection improvement, and lower costs.
At present, there are only two published studies comparing LDR vs. HDR boost in prostate cancer, both including patients of intermediate- and high-risk [24, 25]. In King et al. [24] (18,403 patients of the National Cancer Database treated with BT boost), the results in terms of OS were similar without comparing toxicities, although they were based on retrospective inhomogeneous series, which the authors were aware of. For all patients analyzed, there were no differences between LDR and HDR boost, when sub-groups were divided by Gleason score, clinical T stage, NCCN risk category, or ADT. In a series of Slevin et al. [25] (287 patients of an institutional database), HDR boost was more than twice as likely to experience biochemical progression compared with LDR boost. At 5 years, bPFS was estimated to be 90.5% for the LDR-EBRT cohort, and 77.6% for the HDR-EBRT cohort. Cumulative incidence of ≥ grade 3 GU and GI toxicities for the LDR-EBRT and HDR-EBRT cohorts were 8% vs. 4% and 5% vs. 1%, respectively, although not statistically significant.
Even though, intermediate- and high-risk prostate tumors have different behaviors, both groups are treated equally with the same therapeutic intensity in most of the published studies, in which BT together with EBRT, constitute the therapeutic program. The use of different BT techniques, total dose, and dose per fraction, among others, are the data, which may influence clinical results and toxicity profiles. Analyzing published series with both risk groups, only three unique prospective randomized studies have been published comparing BT boost with EBRT [21, 23, 26]. All three were analyzed in a meta-analysis published in 2018, describing a significant benefit of 5-year biochemical control in favor of patients, for whom BT was used as a boost [27] being recommended for IR patients. For Sathya et al. [21], bPFS at 10 years was 67% in BT arm (LDR temporary 192Ir), for Hoskin et al. [23] analyses it was 46% (HDR temporary 192Ir), and in ASCENDE-RT trial [26] (LDR permanent 125I) it was 83% at 9 years. These three different studies have different total EBRT doses and even fractionations, with the first two employing exclusive EBRT doses much lower than current standards. In addition to the above, randomized data from mature mono-institutional series with a long follow-up reports have been published with very favorable results for patients treated with BT as a boost. All of them described high bPFS rates, between 84% and 91% in IR patients using either HDR [28-33] or LDR [34].
The published literature of combined treatment (EBRT plus LDR or HDR-BT) including IR patients exclusively is scarce [35-38]. In contrast to these data, our series reflects great homogeneity, both in the performance of the treatments and in the follow-up of these patients over time. Furthermore, all the treatments as well as clinical and technical data compilations were carried out by the same team. This provides uniformity in the criteria used in the handling of the data, eliminating “inter-observer” variables and delivering greater rigor to the observation. These data showed no differences when LDR-BT or HDR-BT was used, obtaining a bPFS of 92% at 90 months with both techniques, which are very similar to 91% at 5 years reached by Martell et al. [35] with HDR, and 87% at 5 years by Chao et al. [36] with LDR.
There are difficulties and limitations in comparing our toxicity with published studies regarding the inhomogeneity of brachytherapy technique itself (real-time, CT planning, MRI fusion), different fractionations of EBRT, and differences in score systems (CTCAE, LENT-SOMA, RTOG scale). Even within the identical system, the events and terminology are different according to the version used (CTCAE v 4.0 or v3.0). The vast majority of our patients reported no side effects or grade 1-2, which corresponds to irritative voiding symptoms treatable by α-blockers or anticholinergic therapy.
Two, very relevant studies, have been published on the implication of BT in a combined treatment of PCa. The first of Hoskin et al. [23] with HDR, and the second, the ASCENDE-RT trial [39] using LDR as a boost. Both are cited as examples in the discussions of multiple publications with negative criticism regarding toxicity. In their study, Hoskin et al. employed an adapted version of a Dische scales for relating toxicity [23]. In the prospective study ASCENDE-RT, Rodda et al. [39] utilized a modified LENT-SOMA criteria, a system that includes multiple adverse effect factors in the same group without being broken down individually. Most of these factors would be classified at a lower degree if other toxicity scales were used. This is evident from the results not reproduced in prospective or retrospective studies, using the same doses in combined treatments.
We have searched publications, in which toxicity was defined based on CTCAE version 4.0 and patients were treated with similar schedules, finding equivalent results to our series. Sakurai et al. [40] analyzed 124 patients who underwent 13 Gy HDR-BT followed by EBRT (46 Gy/23 fractions), with the reported incidences of grade 2 urinary frequency and urinary retention in 45.5% and 18.1%, respectively, and 3.3% of grade 2 rectal hemorrhage, as evaluated by lower gastrointestinal endoscopy observed in four patients. Büchser et al. [41] analyzed prospectively 210 patients treated with HDR-BT (15 Gy single fraction) plus three-dimensional conformal radiotherapy (3D-CRT) (37.5 Gy/15 fractions). The incidence of toxicity was very low. Grade 2 events were due to urethral strictures (3 patients), incontinence (3 patients), hematuria (2 patients), and retrograde ejaculation (1 patient). The incidence of late grade 2 and grade 3 GI toxicity was 5.2% (11 patients) and 1% (2 patients), respectively. The authors concluded that the reason for these very low toxicities were partly explained by the use of real-time MRI-TRUS fusion for treatment planning. Kollmeier et al. [42] analyzed safety and toxicity utilizing LDR 103Pd prostate brachytherapy in combination with ultra-hypofractionated radiotherapy (25 Gy delivered in 5 Gy fractions) in 46 patients with intermediate-risk prostate cancer. The most common grade 2 urinary toxicities investigated were urinary frequency/urgency (25%) and urinary obstruction/retention (10%). No patients suffered from urinary retention requiring catheterization. Chao et al. [36] reported retrospectively 31 patients who received 45 Gy plus 125I boost with an incidence of late grade 3 GU toxicity of 6.5% with urinary retention, with two patients requiring either a bladder neck incision (BNI) or TURP.
In the present study, despite the differences between the two groups (greater frequency of previous TURP and adenectomy, higher percentage of comorbidities, a three-fold greater use of anticoagulants, larger prostate size in the HDR-BT boost patients and higher EBRT doses), no differences between them in terms of toxicity grade 2 or higher were noted. These aspects may explain the significant increase in grade 1 GU toxicity and grade 1 rectal bleeding when HDR was used, not requiring treatment or being controlled with medication in all cases. We described 3.5% of urethral stenosis grade 3 that needed an elective operation, which was in the lower range of the published literature, as previously stated.
The only statistically significant factor in biochemical control was the differentiation between FIR and UIR. When we analyzed both groups separately, we found a 10-year clinical and biochemical control in 100% of the patients with FIR, and patients with good prognosis factors, for which this data greatly improved the results obtained with exclusive treatments: EBRT, LDR, or HDR monotherapy [43-47].
Existing data differentiating patients into FIR and UIR are the key factor in prognosis, outcomes, and treatment selection. Patients with unfavorable intermediate-risk disease had a higher risk of PSA recurrence, local failure, development of metastases as well as death from prostate cancer, as described by Preisser et al. [48]. Due to this, a combined treatment of external beam and brachytherapy (± androgen deprivation) is recommended [1].
In our series, bPFS in UIR was 85% at 90 months; biochemical failure was evident in 10 patients, 4 treated with LDR-BT and 6 with HDR, without statistically significant differences in terms of recurrence (lymph node or distant) and technique employed. Only one patient treated with seeds had a local failure. ADT addition, showed an increased bPFS in UIR patients [49, 50]. In this homogeneous mono-institutional series, 99% of the patients received at least 6 months of ADT. None of the factors analyzed independently, including Gleason score 7 (4 + 3), > 50% of positives biopsies, perineural invasion, or 2-3 factors that define IR group, showed a statistical difference leading to greatest number of biochemical failures in this group. We hypothesize that the percentage of failures may be due to other technical aspects (total dose, no pelvic irradiation) or biological (genetic mutations) factors. A very important difference, when comparing our data with the outcomes of published series, is the wide difference of schemes in both treatment components (BT and EBRT), differences in doses and sometimes in volumes of treatments, such as 15 Gy combined with 37.5 Gy in 15 fractions [35, 51], 60 Gy with 9 Gy with HDR [45], 46 Gy plus 2 fractions of 11.5 Gy [33], 20/44 Gy combined with 103Pd boost [52], or 46 Gy plus 115 Gy 125I implant in the ASCENDE-RT trial [26]. Inevitably, this diversity represents a notable difference between the various “intensities” of respective programs, which, in our department, led us to consider that perhaps the dose selected in 2013 in combined treatments with HDR (60 Gy with EBRT and 10 Gy) could be insufficient for patients with unfavorable intermediate-risk, and consequently, modifying the protocol based on the results of this analysis, adapting it to the more recent published recommendations [1].
Potential limitations of this study are the retrospective analyses and mono-institutional nature. It seems appropriate to emphasize the performance of more prospective studies in order to identify significant factors in the results, in an attempt to achieve greater control and more stable results in this particular group of patients.
Conclusions
In this mono-institutional and very homogeneous study, despite being retrospective, it is demonstrated that BT combined with EBRT is an excellent therapeutic option in patients with IR PCa, with equal results when both LDR and HDR techniques are employed, showing very low toxicities.
Patients included in UIR constitute a different entity, and should be treated as patients with high-risk factors. The stratification and identification of both risk groups is of great importance.


