Introduction
Food allergy (FA) has become a health concern worldwide. The number of people suffering from FA is constantly increasing, especially in highly developed countries. The World Allergy Organization estimated the prevalence of FA in 2013 to be 11–26 million people in Europe, and, potentially, 220–520 million people worldwide [1]. The rise over the last decades is known as the second wave of the allergy epidemic [2]. Food sensitization (FS), apart from other features expressed as the presence of allergen-specific antibodies, is a prerequisite for development of FA. Patterns of sensitization to food allergens have previously been studied in children to better understand allergy development [3]. However, the results available in the literature differ between countries, what is mainly due to the different clinical manifestations of allergy, diagnostic criteria and methods used [4]. A European study by Lyons et al. revealed the substantial geographical variation in the prevalence of FS in school-age children across Europe, providing estimates for 24 commonly implicated foods in multiple countries [5].
The detection of allergen sensitization is based on in vivo and in vitro tests. Briefly, in vitro tests detect total and specific IgE in peripheral blood [6, 7], whereas in vivo skin prick test reflects the functional role of these antibodies in type I immediate allergic reaction [8–10].
Component-resolved diagnostics (CRD) offers new possibilities in the evaluation of FA. It allows simultaneous determination of specific IgE against hundreds of native and recombinant allergens and differentiation of primary sensitization from cross-reactivity [11]. This is particularly important in case of plant-derived foods as pollen-associated cross-reactivity may play a role in the misdiagnosis of food allergy.
In this study, CRD analysis using ALEX platform was used for the safe and accurate detection of allergen sensitization in children. Prevalence of FS and FA differs depending on geographical location and dietary habits. However, in many countries, including Poland, these habits were changed since other foods, non-typical for the region, were introduced to Polish cuisine. The manifestation and severity of food hypersensitivity are extremely variable and depend on the type of ingested food. Numerous patients are sensitized although completely asymptomatic, some of them may show only local reactions, as contact urticaria or oral allergy syndrome (OAS), whilst others may present more serious symptoms and systemic reactions, including vomiting, abdominal pain, urticaria-angioedema, asthma attack, up to anaphylactic shock.
Allergies to nuts (tree nut and peanut) usually start in childhood, but unlike other food allergies, they rarely fade away and generally persist throughout life [12]. This type of allergy is especially dangerous due to the physical and chemical characteristics of main nut allergens. They belong to a group of seed storage proteins and are heat- and digestion-resistant [13]. They are frequent causes of serious allergic reactions resulting in death in both children and adults [14]. The knowledge concerning the sensitization profile to nut allergens in children is clinically relevant since it enables the use of secondary prophylaxis to prevent the development of allergy and its complications.
Aim
The aim of this study was to investigate the rates and patterns of sensitization to nut extracts and/or their components in the children population from central Poland, tested for suspected food allergy, including nut allergy.
Material and methods
A retrospective assessment involved anonymized clinical and laboratory records retrieved from the medical database of patients, who were admitted to the Department of Pediatric Pneumonology and Allergy, Medical University of Warsaw, between July 2018 and March 2021. The selection criteria for the database search included both sexes, age 0–18, available medical history of the patient, clinical symptoms, suspected IgE-mediated allergy, and laboratory data for total and specific IgE.
The data concerning patient’s status of specific allergen sensitization originated from multiparametric assay Allergy Explorer2 (ALEX2) (Macro Array Diagnostics, Vienna, Austria). This method enables acquisition of a specific IgE profile comprising 221 reagents (104 allergen extracts and 117 components), providing the broad screening of potential allergens.
In this particular study we have focused on extracts from 8 nuts and 16 different components available from 3 nuts – hazelnut, peanut and walnut. For the selection criteria used for this study, the specific IgE concentration of 0.3 kUA/l or higher was arbitrarily defined as positive.
The study protocol was reviewed and approved by the Ethics Committee at the Medical University of Warsaw (AKBE/81/2022).
Results
Baseline characteristics of study population
Based on inclusion criteria applied for database search the medical data of 598 patients were used for further assessment. The selected study population comprised of 217 (36%) females and 381 (64%) males. The mean age of study subjects was 6.8 years, with the median 6.0. To facilitate further assessment the study population was divided into four age-related sub-groups: the youngest children – 0–2 years old (22%), toddlers – 3–5 years old (26%), school children – 6–12 years old (35%) and teenagers – 13–18 (16%). Regarding the clinical status of allergy, the majority of children (72%) from the study population had been diagnosed with food allergy, 52% of patients suffered from allergic rhinitis (AR), 42% of patients were diagnosed with atopic dermatitis (AD), and 29% of children had asthma. Noteworthy, 36% of children had the history of at least one anaphylaxis. Food allergy was predominant in children under 6 years of age. It affected 91% of individuals in the youngest group but that number gradually decreased to nearly 55% in the group of teenagers. The percentage of patients with a history of anaphylaxis was similar in all age groups ranging from 31 to 41%. The baseline characteristics of the study population was shown in Table 1.
Table 1
Characteristics of the study population
| Diagnosis | Patient number | Age group | Sex | ||||
|---|---|---|---|---|---|---|---|
| 0–2 | 3–5 | 6–12 | 13–18 | Female | Male | ||
| 598 | 131# (22%) | 157# (26%) | 212# (35%) | 98# (16%) | 217# (36%) | 381# (64%) | |
| Asthma | 173* (29%) | 14 (8%*/11%#) | 32 (18%*/20%#) | 87 (50%*/41%#) | 40 (23%*/41%#) | 63 (36%*/29%#) | 110 (64%*/29%#) |
| Allergic rhinitis | 312* (52%) | 16 (5%*/12%#) | 79 (25%*/50%#) | 148 (47%*/70%#) | 69 (22%*/70%#) | 107 (34%*/49%#) | 205 (66%*/54%#) |
| Atopic dermatitis | 249* (42%) | 70 (28%*/53%#) | 59 (24%*/38%#) | 93 (37%*/44%#) | 27 (11%*/28%#) | 87 (35%*/40%#) | 162 (65%*/43%#) |
| Food allergy | 433* (72%) | 119 (27%*/91%) | 118 (27%*/75%#) | 143 (33%*/67%#) | 53 (12%*/54%#) | 164 (38%*/76%#) | 269 (62%*/71%#) |
| Anaphylaxis | 216* (36%) | 41 (19%*/31%) | 64 (30%*/41%#) | 75 (35%*/35%#) | 36 (17%*/37%#) | 89 (41%*/41%#) | 127 (59%*/33%#) |
Prevalence of sensitization to nut allergens
The assessment of data from ALEX assay has shown that the prevalence of nut allergen-specific antibodies differed in regards to the nut species, but also varied depending on the age of tested patients. Among antibodies directed against nut allergens, those specific for hazelnut and peanut molecules were the most prevalent. They were detected in more than half of patients from the entire group, in 56% and 55% of individuals, respectively. They were followed in that comparison by IgE against cashew, which was detected in 37% of cases, whereas antibodies specific to other nuts, including walnut (23% of children), were less frequent, below 25% of cases.
The assessment in pre-selected age-related groups has shown that the sensitization to peanut and hazelnut molecules were the most common in school children, affecting over 60% of cases each. Except for the youngest group the hazelnut sensitization was predominant in every age, and its prevalence increased with age, from 41% of youngest children, to almost 70% in school children, and then decreased to 52% in teenagers. Oppositely, the peanut sensitization was detected in more than 52% of individuals from all groups except for teenagers, where it concerned 44% of cases.
Cashew sensitization was found in over one third of the patients, with the highest prevalence among the youngest children, accounting for almost half of them, and then gradually decreased to 16% in teenagers. Macadamia-specific antibodies were the least common (13% of all cases), in youngest children and in teenagers, they were detected in less than 10% of patients (Table 2).
Table 2
Prevalence of sensitization to nut types
| Parameter | Hazelnut | Peanut | Cashew | Pecan nut | Almond | Brazil nut | Walnut | Macadamia |
|---|---|---|---|---|---|---|---|---|
| Patient group (n = 598) | n = 333# | n = 326# | n = 219# | n = 163# | n = 145# | n = 140# | n = 139# | n = 80# |
| 0–2 (n = 131)* | 54 (41%*/16%#) | 71 (54%*/22%#) | 64 (49%*/29%#) | 32 (24%*/20%#) | 44 (34%*/30%#) | 46 (35%*/33%#) | 24 (18%*/17%#) | 12 (9%*/15%#) |
| 3–5 (n = 157)* | 84 (53%*/25%#) | 82 (52%*/25%#) | 57 (36%*/26%#) | 38 (24%*/23%#) | 31 (20%*/21%#) | 29 (18%*/21%#) | 34 (22%*/24%#) | 18 (11%*/22%#) |
| 6–12 (n = 212)* | 144 (68%*/43%#) | 130 (61%*/40%#) | 82 (39%*/37%#) | 70 (33%*/43%#) | 60 (28%*/41%#) | 54 (25%*/38%#) | 61 (29%*/44%#) | 41 (19%*/51%#) |
| 13–18 (n = 98)* | 51 (52%*/15%#) | 43 (44%*/13%#) | 16 (16%*/7%#) | 23 (23%*/14%#) | 10 (10%*/7%#) | 11 (11%*/8%#) | 20 (20%*/14%#) | 9 (9%*/11%#) |
| Female | 114 | 112 | 76 | 57 | 42 | 48 | 45 | 23 |
| Male | 219 | 214 | 143 | 106 | 103 | 92 | 94 | 57 |
| Asthma (n = 173)* | 124 (72%*/37%#) | 115 (66%*/35%v) | 86 (50%*/40%#) | 69 (40%*/42%#) | 55 (32%*/38%#) | 54 (31%*/38%#) | 54 (31%*/39%#) | 39 (22%*/49%#) |
| Allergic rhinitis (n = 312)* | 224 (72%*/67%#) | 207 (66%*/63%#) | 115 (37%*/52%#) | 102 (33%*/62%#) | 75 (24%*/52%#) | 73 (23%*/52%#) | 84 (27%*/62%#) | 52 (17%*/65%#) |
| Atopic dermatitis (n = 294)* | 159 (54%*/48%#) | 159 (54%*/49%#) | 121 (41%*/55%#) | 94 (32%*/58%#) | 86 (29%*/59%#) | 84 (28%*/60%#) | 79 (27%*/57%#) | 51 (17%*/64%#) |
| Food allergy (n = 433)* | 275 (63%*/82%#) | 277 (64%*/85%#) | 202 (47%*/92%#) | 147 (34%*/90%#) | 132 (30%*/91%#) | 127 (29%*/91%#) | 128 (29%*/92%#) | 74 (17%*/92%#) |
| Anaphylaxis (n = 216)* | 135 (62%*/40%#) | 131 (61%*/40%#) | 115 (53%*/52%#) | 80 (37%*/49%#) | 69 (32%*/47%#) | 71 (33%*/51%#) | 63 (29%*/45%#) | 32 (15%*/40%#) |
Sensitization to hazelnut molecules
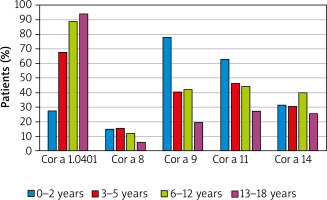
The assessment of a sub-group of 333 patients sensitized to hazelnut allergens revealed that 74% of them had specific antibodies against pathogenesis-related (PR)-10 protein Cor a 1.04. The other allergenic components responsible for sensitization were: storage proteins – Cor a 11 (found in 45% of sensitized patients), Cor a 9 (44% of cases), Cor a 14 (34% of sensitized children), and the lipid transfer protein (LTP) – Cor a 8, detected in 12% of individuals.
The majority of hazelnut-sensitized children (61% of cases) had IgE specific against one of the storage proteins, however, monosensitization to them was scarce, whereas co-sensitization to Cor a 9 and Cor a 14 components was detected in 23% of cases. In contrast to that, monosensitization to Cor a 1.04 component was observed in nearly 40% of hazelnut-sensitized patients.
When assessed in regards to the age groups of sensitized individuals, the sensitization to Cor a 1.04 has appeared the most prevalent, which was observed in the vast majority of school children and teenagers sensitized to hazelnut (89% and 94%, respectively), as well as in toddlers (almost 70%). Noteworthy, except for the youngest group with 28% of sensitized cases, predominance of sensitization to Cor a 1.04 molecule over the other components was obvious. In the youngest patients storage proteins Cor a 9 and Cor a 11 appeared the main allergens (78% and 63%, respectively). Interestingly, the occurrence of Cor a 1.04 sensitization in the assessed sub-group significantly increased with the age, whereas in case of Cor a 9 and Cor a 11 the opposite trends were observed (Figure 1).
Sensitization to peanut molecules
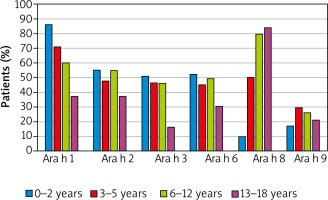
The detailed profile of IgE antibodies specific against main peanut molecules was analysed in the sub-group of 326 peanut-sensitized children. The main molecule involved in peanut sensitization is storage protein Ara h 1, which induced specific antibodies in 65% of peanut-sensitized patients. The antibodies specific for other molecules, found in peanut-sensitive individuals, included PR-10 protein Ara h 8 (57% of cases), other storage proteins – Ara h 2 (51%), Ara h 6 (46%) and Ara h 3 (43%) as well as LTP Ara h 9 (24%).
Specific IgE antibodies against at least one of the storage proteins were detected in 72% of peanut-sensitive patients. Ara h 1 and Ara h2 polysensitization was observed in 46% of sensitized children, whereas polysensitization to all storage proteins was detected in more than 1/3 of patients. Monosensitization to individual storage proteins or LTP Ara h 9 was relatively rare. Interestingly, 21% of peanut-sensitive children have had antibodies specific to PR-10 Ara h 8 as the only peanut component.
The most frequent (86% of cases) was sensitization to Ara h 1 in the youngest children. However, the percentage of individuals sensitized to that molecule gradually decreased to the level below 40% in teenagers. Oppositely, specific IgE antibodies against Ara h 8 were detected in only 10% of youngest patients, but in almost 80% of school children and teenagers (Figure 2).
Sensitization to walnut molecules
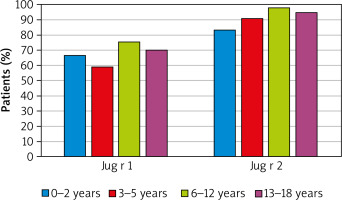
Almost each patient (94%) from the sub-group sensitized to walnut (n = 139) had IgE antibodies against the storage protein Jug r 2. The antibodies against another storage protein, Jug r 1, were detected in nearly 70% of walnut-sensitized patients, whereas 63% of patients were sensitized to both components.
When assessed in regards to the age, the prevalence of antibodies to Jug r 2 component in walnut-sensitized patients only slightly varied among all age groups, nevertheless, in all of them it was very high, above 80%. Similarly, the occurrence of sensitization to Jug r 1, although seemingly lower, compared to that of Jug r 2, in all age groups remained on the similar level of approximately 60–70% (Figure 3).
Co-sensitization to nuts
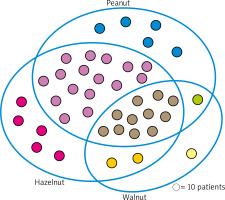
The assessment of IgE antibodies profile against various nuts has shown that 398 individuals (67% of the whole group) were sensitized to at least one of above-mentioned – hazelnut, peanut or walnut. Noteworthy, 109 children (27% of that sub-group) were sensitized to only one nut, 178 (45%) children were co-sensitized to different two nuts, whereas 111 individuals (28% of the sub-group, and 18% of all assessed cases) revealed simultaneous co-sensitization to all three nuts. 81% of hazelnut-sensitized patients revealed co-sensitization to peanut, and almost 38% of them were co-sensitized to walnut. Among all analysed cases they constituted approximately 45% and 21%, respectively. Co-sensitization to walnut in peanut-sensitized children concerned 35% of individuals, which corresponded to 19% of the whole group (Figure 4).
Discussion
Component-based diagnostics was proven to be an important tool in the investigation of patients with a suspected nut allergy, in particular to differentiate between primary and cross-reactive sensitization. To our best knowledge, our report is the largest data set that summarizes clinically relevant data concerning sensitization profiles of children from central Poland, diagnosed for suspected nut allergy. Since the detailed identification of main allergens, responsible for sensitization in children from various age groups gives the opportunity to introduce more effective prophylaxis, it may help to avoid life-threatening complications, including anaphylactic shock and death. Below we shortly discuss some practical aspects of our findings.
Hazelnut sensitization
Hazelnut allergen Cor a 1.04 reveals a high degree of structural homology to PR-10 proteins from the pollens of the Fagales order (e.g. birch, hazel or alder). The predominance of specific allergens appears to be strictly related to the geographical origin of the allergic subjects and likely reflects exposure to different pollens. As the main deciduous tree of the central Poland region is birch, hence a high degree of sensitization to the main birch allergen Bet v 1 (PR-10) is observed very often. Therefore, a high prevalence of sensitization to the Cor a 1.04 molecule is also observed in birch endemic regions. Our data are convergent with results published in 2020 by Lyons et al. concerning prevalence of food sensitization in children [5]. They have found PR-10 sensitization in 47.8% to 52.2% of plant-source food-sensitized children from the birch-endemic European countries, including Switzerland, the Netherlands, Lithuania and Poland.
Another clinically relevant allergen in hazelnut-sensitized children is the heat- and digestion-resistant LTP molecule – Cor a 8. Noteworthy, sensitization to this molecule usually results from primary sensitization to other LTPs. Thus, in central and north-central Europe, the Art v 3 molecule, LTP from mugwort [15], whereas in the southern part of Europe, in the Mediterranean region, Pru p 3, LTP from peach, have been implicated as main primary inducers of non-specific sensitization to LTP [16]. Despite a relatively low frequency in children population (in our data approx. 10%), the clinical relevance of Cor a 8 is outstanding as it may be associated with anaphylactic reactions, especially in the presence of some co-factors (e.g. exercises or stress).
Peanut sensitization
Ara h 1 (7S globulin), Ara h 2 (2S albumin), Ara h 3 (11S globulin) and Ara h 6 (2S albumin) are highly abundant, structurally stable seed storage proteins of peanut. Clinical relevance of the precise identification of sensitization to these molecules is indisputable, since IgE specific to them have been associated with severe systemic symptoms [17]. Moreover, as found in our study, sensitization to one of storage proteins, Ara h 1, was the most prevalent in the youngest children (86%), and although its frequency decreased with age, even in the teenagers group remained relatively frequent.
The sensitization to Ara h 2 was less prevalent than that to Ara h 1 and comparable to the remaining storage proteins – Ara h 3 and Ara h 6. Similarly to them, it concerned almost half of peanut-sensitized children, except for the teenagers group, where the frequency of specific antibodies was slightly lower. Importantly, the assessment of patients’ clinical history has revealed some association between the prevalence of sensitization to Ara h 2 molecule and a history of anaphylaxis, which was the highest in the group of school children (data not shown). This observation could support the thesis that the detection of IgE specific to Ara h 2 may enhance diagnostic specificity and can be useful in discriminating between peanut allergic and tolerant individuals [18, 19].
A gradual age-dependent change in the prevalence of sensitization, especially to Ara h 1, but also to other storage proteins (Ara h 2, -3 and -6), observed in our study, is compatible with the American data by Valcour et al., were the frequency of sensitization to the storage protein decreased with age, too [20]. The possible explanation of this phenomenon could be the gradual loss of sensitization to peanut storage proteins over time, but this straightforward hypothesis requires further verification.
On the other hand, an alternative explanation could be an effect of “dilution”. In the early childhood, the prevalence of antibodies specific to storage proteins reflects the primary sensitization to peanut allergens. In the group of older children, presumably the decreasing frequency of those antibodies results from including in this group of some patients, who, although primarily non-sensitized to peanut storage proteins, reveal serologic cross-reactivity with other peanut molecules. That cross-reactivity may be due to primary sensitization to birch PR-10 homolog Bet v 1, which mimics Ara h 8, or less frequently, to mugwort LTP Art v 3 that mimics Ara h 9. Hence, the “misinclusion” of such individuals will result in the apparent shift in the sensitization profile of older groups.
The abovementioned concept may further be supported by the observation regarding the sensitization to PR-10 protein – Ara h 8. Interestingly, the prevalence of IgE antibodies specific to this molecule represented a quite opposite age-related tendency to that of Ara h 1. As previously mentioned, the low frequency of sensitization to Ara h 8 in early childhood, and its gradual, significant increase observed in older groups, may actually result from the contribution by cross-reactive antibodies to Bet v 1 in patients primarily sensitized to birch pollen. Indeed, the increased prevalence of sensitization to Ara h 8 in teenagers corresponded to high frequency of birch pollen allergy diagnosed in that group (data not shown).
Walnut sensitization
According to data from the EuroPrevall study, approximately 3% of the European adults were predicted to be sensitized to walnut, with a range from 0.1% in Iceland to 8% in Spain [21]. Walnut allergens have been recognized as important elicitors of food allergy in sensitized patients with a high frequency of severe reactions. Among children and adolescents, walnut allergens belong to the most important triggers, accounting for 16% of cases of the anaphylaxis to tree nuts [22].
Two storage proteins, Jug r 1 (2S albumin) and Jug r 2 (7S globulin), were used in our study to identify patients with primary sensitization to walnut. The sensitization to these molecules was relatively high and similar in all age groups, with the mean rate of approx. 65% for Jug r 1 and nearly 90% for Jug r 2.
Noteworthy, sensitization to Jug r 1 was more frequent in patients with the early onset of food allergies. This finding reflects a prominent role of storage proteins in paediatric subjects with primary sensitization, which is usually acquired in childhood and is associated with severe systemic reactions [23].
However, the role of Jug r 2 should be considered with some caution since, despite low homology between this molecule and main peanut storage protein - Ara h 2, antibodies against them reveal strong cross-reactivity, which may interfere with proper diagnosis [24, 25].
Regrettably, due to some technical reason, our assessment did not include IgE specific to other walnut molecules, especially LTP Jug r 3 (cross-reactivity with peach Pru p 3 and mugwort Art v 3) or PR-10 homolog Jug r 5 (cross-reactivity with birch Bet v 1).
Our results are consistent with other literature data, where children with tree nut allergy have an increased risk of co-sensitization to other nuts [26, 27]. The co-sensitization rate to tree nuts is relatively high, especially since certain combinations (e.g. walnut with pecan, or cashew with pistachio nuts) appear more commonly, reaching up to 86% [28]. The clinical relevance of this observation is remarkable as co-sensitization highly increases the risk of life-threatening reactions even after ingestion of products with trace nut contamination (hidden allergens).