Introduction
The last few decades have seen a considerable increase in the prevalence of various forms of allergies, particularly in industrialized, more developed countries [1]. The high incidence rate of food allergies (FA) has contributed significantly to the increased prevalence of allergies in this period [2]. Although the exact prevalence of FA varies by country (mainly due to regional differences in food types and eating habits) foodstuffs are a common trigger of allergic symptoms. FA usually begins in early childhood and in some cases may persist throughout life [3, 4].
The response to food allergens includes a broad spectrum of clinical reactions, ranging from mild urticaria to life-threatening anaphylaxis [5]. The severity and intensity of anaphylaxis depends on the type of food ingested. From a clinical standpoint, the sensitization to peanuts and various tree nuts (e.g. hazelnuts, walnuts, cashew nuts, Brazilian nuts, macadamia nuts, almonds) appears to be particularly dangerous at every stage of allergy development [6]. This is due to the physical and chemical properties of major nut allergens, which are relatively resistant to heat processing and digestion [7]. Detectable amounts of some nut allergens may be found in various food products, either as their intended components, or as the trace contaminations, resulting from technological processes in the food industry [8]. These contaminations may entail increased health risks, especially in groups of sensitized children, who are particularly prone to excessive ingestion of various snacks or other processed meals where such contaminations are more commonly found [9].
Specific immunotherapy is the only method that alters the natural course of allergy, including food allergy. Its use (desensitization) leads to the development of allergen tolerance and, in some patients, to sustained unresponsiveness. However, in case specific immunotherapy is unavailable, the most effective method of allergy management is the prevention of allergen exposure.
The critical safety issue for sensitized individuals is the avoidance of allergenic food ingestion, which, however, requires the proper identification of sensitizing allergens [10].
Beyond food allergy, IgE antibodies specific to nut allergens may also be found in patients with atopic dermatitis (AD), allergic rhinitis (AR), or atopic asthma (AA), especially since atopic diseases frequently appear concurrently [11]. Routinely, sensitization is verified by the detection of allergen-specific antibodies. Since sensitization is not synonymous with allergies, in early stages specific antibodies may be considered as predictors of future disease development, whereas in later stages they are serological markers of advanced allergy.
Aim
The aim of this retrospective study was to assess the patterns of sensitization to selected nuts in four age-stratified groups of children from central Poland, suffering from food allergy with or without accompanying atopic diseases.
Material and methods
The assessment is based on data containing medical records of all consecutive children suspected of IgE-mediated allergy, consulted or treated at the Department of Paediatric Pneumonology and Allergy of the Medical University of Warsaw, in the period between 2018 and 2021.
The initial diagnosis of allergic disease was carried out by general practitioners based on clinical signs of allergy. Patients with suspected IgE-dependent allergy were referred to the clinic for further diagnostics.
The retrospective research used the following inclusion criteria for subjects: age 0–18, both sexes, suspected IgE-mediated allergy with clinical symptoms, availability of the patient’s medical history and laboratory data (including those from the Allergy Explorer2 – ALEX2 – Macro Array Diagnostics, Vienna, Austria). This multiparametric assay enables the simultaneous screening of specific IgE against a broad spectrum of potential allergens, comprising 104 allergen extracts and 117 major allergen components. To specify the inclusion criteria, the concentration of specific IgE ≥ 0.3 kUA/l was considered as positive. The extracted data were anonymized and subjected to further analysis. Among all available component diagnostics, sensitization to common nut allergens, particularly hazelnut, peanut and walnut, was thoroughly evaluated. The study protocol was approved by the Ethics Committee at the Medical University of Warsaw (No. AKBE/81/2022).
Results
Characteristics of study population
The retrospective analysis pertained to anonymized clinical and laboratory records extracted from a medical database, according to criteria specified above. These records included data of 598 patients (381 male and 217 female) with a mean age of 6.8 years (median 6.0 years), which were further divided into four age-related groups:
131 young children (0–2 years old) – 21.9%,
157 toddlers (3–5 years old) – 26.2%,
212 schoolchildren (6–12 years old) – 35.4% and
98 teenagers (13–18 years old) – 16.4%.
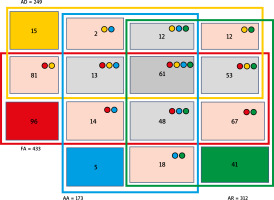
According to the main diagnosis, 433 patients (72.4% of the study cohort) had FA. FA was common in every age group, but in the young children group it was diagnosed in 91% of subjects. Interestingly, only 22.2% of children of the FA group (16.1% of the entire cohort) presented standalone FA, whereas the remaining 77.8% of patients were diagnosed with at least one concomitant atopic disease – AR, AD, or AA. Importantly, 14.1% of children of the FA group (10.2% of the whole cohort) were simultaneously co-diagnosed with either AR, AA or AD (Figure 1). The main symptoms in FA reported by majority of patients included abdominal pain/discomfort with nausea and/or vomiting.
Allergic rhinitis was diagnosed in 52.2% of the entire cohort. However, only 13.1% of children of the entire cohort (6.9% of the study cohort) had AR alone, whereas nearly 87% of them were co-diagnosed with some other atopic disease – FA, AD or AA. The main symptoms in the AR group included nasal itching, sneezing and/or cough.
Atopic dermatitis was identified in 41.6% of the entire cohort. However, only 6% of the AD group (2.5% of the entire cohort) had AD alone, whereas the remaining 94% also suffered from FA, AR or AA. The main symptoms in the AD group included eczema and/or skin itching.
Atopic asthma was diagnosed in 28.9% of children of the entire cohort. Notably, asthma as the sole diagnosis was identified in only 2.9% of individuals from this group, which corresponded to 0.8% of the entire cohort. The vast majority (97.1%) of these patients were co-diagnosed with AR, FA or AD. The main symptoms in the AA group included cough, wheezing and/or dyspnoea.
A history of at least one episode of anaphylaxis was reported in 216 patients (36.1% of the study cohort) and their percentage, ranging from 31% to 41%, was similar in all analysed age groups. The study group also included 60 individuals (10% of the entire cohort), who were not allocated to any of above-described atopic groups. The main symptoms reported during anaphylaxis included urticaria, sneezing, dyspnoea, sleepiness and/or vomiting.
Prevalence of nut sensitization
The antibodies specific to selected nuts were found in 67% of children from the assessed cohort. Interestingly, only 27% of them were mono-sensitized, whereas simultaneous sensitization to two or three nuts was detected in 45% and 28% of children, respectively.
The most common sensitization to hazelnut or peanut in the whole study group affected 56% and 55% of patients, respectively. Notably, approximately 81% of hazelnut-sensitized patients revealed simultaneous sensitization to peanut, whereas 38% of them were co-sensitized to walnut.
When analysed in regard to the diagnosis, sensitization to hazelnut allergens was found in 63% of children with FA and 72% of children with AA or AR, assessed independently of each other. The prevalence of antibodies against peanut in those sub-groups was 64% and 66%, respectively. Among patients, who experienced at least one episode of anaphylaxis, the antibodies against hazelnut or peanut were found in 62% and 61% of individuals, respectively. In patients with AD the prevalence of sensitization to hazelnut or peanut was similar, and accounted for 54% of this sub-group.
The prevalence of sensitization to other nuts was less frequent. Antibodies specific to cashew were found in 37% of children from the whole group, but they accounted for 50% of patients with AA and 47% with FA. Furthermore, 53% of patients with a history of anaphylaxis were also sensitized to cashew allergens. The sensitization to other common nuts, including walnut, pecan, almond and Brazilian nuts, was similar and ranged between 23% and 27%. Similarly, when assessed with respect to patients’ allergy diagnoses, it affected approx. one-third (27% to 40%) of patients with either FA, AD or AA, as well as episodes of anaphylaxis. The lowest occurrence was found in cases of sensitization to macadamia nuts that affected 14% of individuals from the whole group, including 22% of patients from the AA sub-group and 17% each from the FA, AR or AD sub-groups. IgE antibodies specific to macadamia allergens were found in just 15% of patients with a history of anaphylaxis.
Apart from sensitization to nut extracts, the prevalence of IgE antibodies against component allergens of hazelnut, peanut and walnut was analysed in children with food allergy, especially when combined with various forms of atopic allergy.
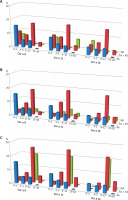
The sensitization to selected hazelnut (Corylus avellana) allergens – Cor a 9, -11 and -14, in 96 children with sole food allergy (sFA) was identified in 26%, 18.8% and 14.6% of cases, respectively, which corresponded to 5.8%, 4.2% and 3.2%, respectively, of the entire FA group. Among individuals with sFA, 60% of children were sensitized to Cor a 9, and 50% sensitized to Cor a 11 in the young children group. Interestingly, no sensitization to the above-mentioned hazelnut allergens was identified among teenagers from the sFA group. Notably, higher frequencies of sensitization to hazelnut allergens were observed in children with FA accompanied by other atopic diseases. For FA overlapping with AD those frequencies accounted for 37.5% (for Cor a 9), 39.4% (for Cor a 11) and 32.2% (for Cor a 14). When calculated for the whole FA group, those values corresponded to 18%, 18.9% and 15.5%, respectively. In patients with FA and AR the respective frequencies accounted for 31%, 35.8% and 28.8%. When calculated for the whole FA group, these values corresponded to 16.4%, 18.9% and 15.2%, respectively. Interestingly, in all above-mentioned combinations, approximately half of cases sensitized to all hazelnut component allergens belonged to the schoolchildren group.
The occurrence of sensitization to Cor a 9 in children with sole AD accounted for 6.7%, for Cor a 11 – 13.3%, and 6.7% for Cor a 14, whereas in children with sole AR alone it was 7.3%, 9.8% and 4.9%, respectively. Unexpectedly, in the group with sole AA alone the sensitization to all aforementioned hazelnut allergens was detected in only one case in the schoolchildren group. That group consisted of only five patients, though (Figure 2).
Figure 2
The occurrence of sensitization to selected hazelnut allergens in age-related groups: young children (0–2), toddlers (3–5), schoolchildren (6–12) and teenagers (13–18). Each graph represents sensitization occurrence in regards to clinical diagnosis: A – sole food allergy – FA (blue bars), food allergy and atopic dermatitis – FA + AD (red bars), atopic dermatitis – AD (green bars); B – sole food allergy – FA (blue bars), food allergy and allergic rhinitis – FA + AR (red bars), allergic rhinitis – AR (green bars); C – sole food allergy – FA (blue bars), food allergy and atopic asthma – FA + AA (red bars), atopic asthma – AA (green bars)
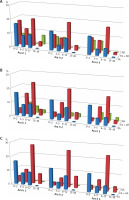
The sensitization to selected peanut (Arachis hypogaea) component allergens – Ara h 1, -2 and -3, in children with sFA was identified in 34.4%, 27.1% and 21.9% of individuals, respectively. Those frequencies, when calculated for the entire FA group, accounted for 7.6%, 6.0% and 4.8%, respectively. Notably, almost half of these individuals belonged to the young children group, whereas no such sensitization was found in the teenagers group.
Interestingly, similarly to the aforementioned sensitization to hazelnut, much higher frequencies of sensitization to component peanut allergens were observed in children, who experienced FA in combination with other atopic diseases. In patients with concomitant AD the specific antibodies against Ara h 1 were found in 52.9% of cases, that corresponded to 25.4% of the whole FA group. These frequencies were similar for individuals with AR (47.2% vs. 24.9% of the whole FA group) and with AA (49.3% vs. 15.5% of the whole FA group). Also, similar sensitization frequencies were found for the remaining two peanut allergens – Ara h 2 and -3. In patients with FA and concomitant AD they accounted for 39.9% and 38.4%, respectively, with concomitant AR – 40.2% and 32.7%, respectively, and with asthma – 41.9% and 38.2%, respectively. Similarly to hazelnut, where FA was combined with other atopic diseases, the sensitization frequency was the highest for all tested peanut allergens in the group of schoolchildren. In that age group, in children with FA and AD it accounted for 19.7% for Ara h 1, 19.2% for Ara h 2 and 18.3% for Ara h 3, in children with FA and concomitant AR it was 23.6%, 21% and 17.9%, respectively, whereas for FA with AA –27.9%, 25.7% and 24.3%, respectively.
The frequency of sensitization to Ara h 1 in children with sole AD accounted for 20%, and for Ara h 2 and Ara h 3 – 13.3% each, whereas in children with sole AR it was 17.1%, 9.8% and 12.2%, respectively. Notably, no cases sensitized to peanut allergens were detected in any individual with sole AA however it should be emphasized that this group included only 5 children (Figure 3).
Figure 3
The occurrence of sensitization to selected peanut allergens in age-related groups: young children (0–2), toddlers (3–5), schoolchildren (6–12) and teenagers (13–18). Each graph represents sensitization occurrence in regards to clinical diagnosis: A – sole food allergy – FA (blue bars), food allergy and atopic dermatitis – FA + AD (red bars), atopic dermatitis – AD (green bars); B – sole food allergy – FA (blue bars), food allergy and allergic rhinitis – FA + AR (red bars), allergic rhinitis – AR (green bars); C – sole food allergy – FA (blue bars), food allergy and atopic asthma – FA + AA (red bars), atopic asthma – AA (green bars)
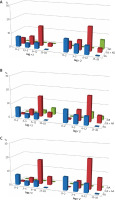
The sensitization to walnut (Juglans regia) component allergens – Jug r 1 and Jug r 2, in the group of sFA affected 14.6% and 21.9% of individuals, respectively. For the entire FA group these values corresponded to 3.2% and 4.8%, respectively. Similarly to aforementioned sensitization to hazelnut and peanut allergens, almost half of sensitized cases with sFA belonged to the young children group, with no cases found in the teenagers group.
The occurrence of sensitization to walnut allergens was much higher in children with FA combined with other atopic diseases. For FA combined with AD it affected 27.4% (for Jug r 1) and 32.7% (for Jug r 2) of children from this sub-group, which corresponded to 13.2% and 15.7%, respectively, of the entire FA group. For FA with AR, the sensitization occurrence accounted for 23.6% and 32.2% of cases, respectively, which corresponded to 12.5% and 17.1%, respectively, of the whole FA group.
In the group of FA combined with AA, the sensitization frequency to Jug r 1 and -2 accounted for 27.9% and 35.3% of individuals, respectively, which corresponded to 8.8% and 11.1% of cases, respectively, for the whole FA group. Again, similarly to hazelnut and peanut sensitization, for both walnut allergens, in all assessed combinations, more than the half of sensitized patients belonged to the schoolchildren group.
In the sole AR group, the sensitization to Jug r 1 (7.3%) and -2 (7.3%) was detected only in schoolchildren and teenagers. In the sole AD group no sensitization to Jug r 1 was found, whereas IgE antibodies specific to Jug r 2 were detected in 6.7% of cases, all from the teenagers group. No sensitization to tested walnut component allergens was detected in the sole AA group (Figure 4).
Figure 4
The occurrence of sensitization to selected walnut allergens in age-related groups: young children (0–2), toddlers (3–5), school children (6–12) and teenagers (13–18). Each graph represents sensitization occurrence in regards to clinical diagnosis: A – sole food allergy – FA (blue bars), food allergy and atopic dermatitis – FA + AD (red bars), atopic dermatitis – AD (green bars); B – sole food allergy – FA (blue bars), food allergy and allergic rhinitis – FA + AR (red bars), allergic rhinitis – AR (green bars); C – sole food allergy – FA (blue bars), food allergy and atopic asthma – FA + AA (red bars), atopic asthma – AA (green bars)
Discussion
Component-based diagnostics is a precise tool used to determine the detailed sensitization profile in atopic children. It enables the introduction of more effective allergen-aimed prophylaxis. Such prophylaxis, when considering also the possible cross-reactive responses, may prevent severe complications, including life-threatening anaphylactic shock or even death [12]. In this report we present the largest, to our best knowledge, dataset focused on sensitization to nuts in atopic children from central Poland. This is followed by a short description of the practical applications of our research.
According to our previous observations, the sensitization to peanut and hazelnut seems to be common in every age group, even in the young children group, affecting almost half of all children diagnosed with any sensitization [13]. Thus, nut allergies may appear in the earliest stages of a patient’s life. The exact pathomechanism of FA development and its link with other atopic diseases remain unclear. Studies on the impact of particular allergens in this process may be affected by possible cross-sensitization or concomitant diseases, especially including atopic ones. Therefore, any analyses should involve individuals exclusively with FA, without any concurrent conditions.
Indeed, when analysed in patients with sFA, despite relatively low absolute values (especially for walnut), the prevalence of sensitization to each evaluated nut allergen was the highest in the young children group, and gradually decreased in older groups. This could suggest the important role of nut sensitization in the early onset of food allergy. Conversely, in patients with sole AD or AR the prevalence of nut sensitization was relatively low in all age groups. Moreover, the sensitization in sole atopic diseases did not reveal any regular patterns, except for the relatively high frequency of sensitization to hazelnut in schoolchildren with asthma alone. However, this finding seems to be an artifact due to a very low number of patients in that group. On the other hand, the repetitive sensitization patterns to hazelnut and peanut, and to some extent walnut, were found in patients with FA and concomitant AD, AR or AA, where the highest sensitization frequency was observed in schoolchildren. Notably, the high similarity in the age-related distribution of sensitization frequency to hazelnut and peanut, observed in all assessed combinations of atopic diseases, was likely due to prevalent co-sensitization to both nuts.
Our observation of two pronounced peaks of sensitization frequency – one in the young children with sFA group and the second, even higher, in schoolchildren with overlapped atopic diseases, aligns with findings published by other authors. Fong et al. reported a correlation between early FA and the development of AR and AA in adulthood [14]. Also, Alduraywish et al. found a relationship between early food sensitization at the age of 6-12 months, and lung dysfunction with reduced forced expiratory volume within 1 s (FEV1) in adolescence [15]. In our study, the high sensitization occurrence shifted from the youngest children with sFA group towards older children with overlapped atopic diseases. This could suggest that although, in general, AR and AA appear later than FA, in many patients they simply follow previous food sensitization.
A relatively low but noticeable frequency of sensitization to peanut and hazelnut was also found in young children with either sole AD or AR. However, especially the latter may result, at least partially, from possible cross-sensitization to birch pollen, which is very common in central Poland. Interestingly, several authors postulated that AD may precede, and significantly increase, the risk of FA. Notably, in the meta-analysis performed by Tsakok et al. the infants with AD revealed a 6-fold higher risk of food sensitization compared to healthy controls [16]. In the same analysis, approx. 53% to 66% of AD subjects were food-sensitized, whereas the symptoms of FA on a challenge were demonstrated in up to 81% of them.
The sensitization to nut allergens is a worldwide issue and nut allergens are the common inducers of anaphylaxis: in children the well-known triggers are peanuts, whereas in young adults, tree nuts [17]. A small Korean study found that in nearly 25% of paediatric patients with anaphylaxis episodes the occurrence of symptoms such as dyspnoea and vomiting was associated with nut sensitization [6]. In a study published by Matias et al., who analysed a small group of children with tree nut allergy, up to 68% of patients experienced an anaphylactic reaction after a first known tree nut ingestion. Notably, in 76% of analysed events the episodes of anaphylaxis were triggered by the ingestion of extremely small nut amounts [18].
In our study the occurrence of anaphylaxis episodes, assessed for the whole group, was similar (31% to 41%) across all age groups. However, when analysed according to allergen sensitization status, it affected up to 62% of children sensitized to hazelnut or peanut, mainly those at school age. This group seems to be particularly at risk of anaphylaxis. It may be due to the highest frequency of sensitization to peanut and hazelnut at this age. However, another reason may be the high risk of unintended exposure to nuts, since children from this age group are the main consumers of various snacks, which frequently are a hidden source of nut allergens.
Although this report presents data from the largest, to our best knowledge, analysis in children from central Poland with suspected food allergies, the relatively low number of individuals with sole atopic diseases seems to be the main limitation of our study. On the other hand, it clearly indicates that sensitization to nuts emerges even in the youngest age groups whereas the frequency of its occurrence almost doubles at school age, together with accompanying atopic diseases. Nevertheless, despite a such “twin-peak” occurrence, the risk of an anaphylactic reaction should be considered serious at any age, especially since in the majority of sensitized patients even the first exposure to nuts may be sufficient to trigger an IgE-mediated hyper-response.