INTRODUCTION
Ambulation, the act of walking, is crucial for performing numerous activities of daily living associated with the categories included in the World Health Organization International Classification of Functioning, Disability and Health (ICF): mobility, self-care, home life, major life areas, and community, social and civic life1.
Walking disorders are common in people who suffer movement diseases associated with the central nervous system (CNS), such as spinal cord injuries (SCI), stroke, traumatic brain injuries (TBI), or multiple sclerosis2. SCI patients can experience paraplegia or tetraplegia, possibly paired with spasticity, resulting in slower gait speed and impaired balance3, Stroke or TBI is associated with hemiparesis, and hence gait asymmetry4,5. Such walking disorders are associated with higher fall risk and lower quality of life6. However, certain parameters, such as gait speed or balance, can act as crucial indicators of the severity of walking deficits and the functional ability to walk in the community7–9.
Functional gait recovery is a common goal for people with CNS movement diseases, and gait rehabilitation typically constitutes a significant portion of the therapy sessions10. A number of interventions have been designed to improve gait function in patients with CNS disorders, and these have demonstrated some level of efficacy. For instance, balance or lower limb strength training might improve walking independence and gait speed11,12. Gait function has been improved by Constraint Induced Movement Therapy (CIMT), a protocol consisting of various ambulation-related activities in which different tasks are repeated based on the shaping principle, the aim being to enhance participation and provide confidence to the patient13. Treadmill training, with and without body weight support, has also been found to be effective in the recovery of walking in different CNS pathologies14,15.
Increasing evidence suggests that high-intensity, repetitive, activity-specific training may enhance gait recovery in patients with CNS disorder14. As such, various robotic gait devices such as the Morning Walk or GEO system have been developed, and these have gained popularity in clinical settings in recent years for the effectiveness and their potential to support the therapist16–18. Moreover, some current clinical guidelines for patients with CNS disorders include the use of robotic devices in combination with conventional therapy to improve gait rehabilitation programs19. However although these devices are used in clinical settings, only Morning Walk has been validated for use in patients with neurological conditions20.
LEXO® is a new end-effector robotic system designed for the rehabilitation of patients with walking disability. It includes a non-immersive virtual reality system a setting that enables the therapist to adjust the degree of assistance or resistance given to the patient. This differentiates it from other end-effector gait systems like Morning Walk or GEO system which just possess a passive mode and do not include virtual reality. However, the effect of the LEXO on walking disability in patients with CNS diseases remains unknown. Therefore, the aim of the present study is to describe the effects of the LEXO end-effector robotic system on functional independence and gait parameters in people with central nervous system disorders.
MATERIALS AND METHODS
Study design
The present work is intended as a descriptive, observational, cross-sectional case series study on the use of the LEXO robotic gait trainer in patients who have suffered injuries to the central nervous system.
Patients were recruited by consecutive non-random sampling based on their attendance at the outpatient Neuron Neurologic Rehabilitation Center in Madrid, Spain. Patients with a prescription of LEXO device sessions as part of their therapy program were invited to participate in the study.
Participants
The study sample consisted of a total of 50 neurologically-impaired individuals, of both sexes. All were recruited between 2020-2021.
The following inclusion criteria were applied: (1) gait disorders caused by any type of central neurological pathology, (2) age over 18 years, (3) ability to ambulate 10 meters with or without assistance of a third person, (4) height at least 120 centimeters. The exclusion criteria comprised (1) scores < 24 points on the Mini Mental State screening test, (2) suffering from acute cardiorespiratory conditions and without consent from a physician, (3) a pain score higher than 6 on the Visual Analog Scale, standing or seated, (4) severe motor or sensory aphasia that impedes comprehension, (5) severe visual-perceptual disturbances, (6) advanced osteoporosis with risk of fracture, (7) acute lower limb fracture, (8) unresolved acute circulatory problems such as thrombophlebitis, (9) orthostatic stress problems with risk of falls, (10) compartment syndrome in lower limbs, (11) anatomical-congenital structural alterations of the lower extremities (anatomical short legs).
All patients gave their written informed consent prior to study participation. This is in accordance with Law 41/2002, November 14, 2002, regulating patient autonomy and the rights and obligations regarding information and clinical documentation, and with the Declaration of Helsinki for ethical principles for medical research involving human subjects. The study protocol was approved by the Ethics Committee of the University Rey Juan Carlos on January 20, 2020 (2110201914719).
LEXO system description
The LEXO robotic gait trainer (Tyromotion, Austria) (Figure 1) is an end-effector system consisting of an electrically actuated movement mechanism that supports passive and active walking. It is designed to support the rehabilitation of patients with walking disabilities caused by neurological damage of the central or peripheral nervous system, such as stroke, traumatic brain injury or spinal cord injury, or orthopedic indications. It offers low set-up times thanks to its end-effector design and the potential to transfer the patient through a lifter system. In addition, personalized adaptation and support is made possible by dynamic body weight support with an active pelvic guidance system, allowing lateral and vertical displacement and stabilization of the trunk. The level and type of support, total practice time, number of steps, step length, walking speed or distance are adjusted with a separate control unit. The unit itself was designed to emphasize active training and promote active participation and motor decision-making by the patient through gamification and a non-immersive virtual environment. The system provides continuous feedback during the session and a summary at the end of each session.
Outcomes
Primary outcome
The primary outcome measure was functional independence, measured with the Functional Assistance Measure (FIM) and the Barthel Index (BI).
The FIM comprises 13 motor items and five cognitive items, for which the participant’s independence in performing the task is recorded on a scale of 1 (total assistance) to 7 (complete independence)21,22. A total score under 42 points indicates severe dependence, while a score of 43 to 85 indicates moderate dependence and more than 85 points total or near-total independence23.
The BI includes ten activities of daily living (ADLs): dressing, grooming, washing, eating, transferring, walking, climbing stairs, urination, defecation, and using the toilet. The total score ranges from 0 to 100 points, with higher scores indicating greater functional independence24,25. A score between 0 and 20 points is related to total dependence, 21 to 60 refers to moderate dependence, and 61 to 90 indicate mild dependence. A score over 91 points reflects total independence26.
Secondary outcomes
Quality of life was measured with the Euroqol-5D EQ-5D questionnaire and the Stroke Impact Scale (SIS). The EQ-5D is a self-assessed questionnaire that evaluates five dimensions: mobility, self-care, ADLs, pain / discomfort, and anxiety / depression. The total score index ranges from 2 (best health) to -2 (worst health), with higher scores indicating better health-related quality of life27,28. The SIS is a self-reported measure aimed at understanding the impact of stroke. It contains eight domains: strength, hand functionality, basic and instrumental activities of daily living, mobility, communication, cognitive ability, emotional status, and participation. The total score ranges from 0 to 295, with higher scores indicating a higher quality of life29.
Walking capacity and endurance were measured with the 6-Minute Walk Test (6MWT). In healthy subjects, the 6MWT ranges from 400 to 700 meters, with a higher score indicating better performance30. Walking more than 312 m is indicative of unlimited community ambulation in neurological conditions31.
Fall risk was measured with the Time Up and Go (TUG) test. A healthy person usually completes the task in 10 seconds or less, with a lower score indicating better performance32. In neurological conditions, completing this test in more than 12 seconds is related with a higher risk of falling33.
Walking speed, cadence, and stride length were measured with PABLO gait assessment equipment (Tyromotion, Austria). The device consists of two inertial sensors placed on either foot of the patient, which are used to measure walking speed, cadence, and stride length while walking 10 meters overground.
Safety was estimated based on the occurrence of severe adverse events related to the LEXO device. A serious adverse event was assumed to be an adverse event that results in death, is life-threatening, requires hospitalization, or results in a disability or incapacity.
Procedures
Prior to any assessments or treatments, all participants provided written informed consent in accordance with Law 41/2002, which regulates patient autonomy and the rights and obligations regarding clinical documentation. The study was performed in accordance with the Declaration of Helsinki for medical research involving human subjects. After obtaining informed consent, baseline assessments were conducted to evaluate functional status, quality of life, walking endurance, fall risk, and gait parameters. These assessments were carried out using standardized protocols to ensure consistency and reliability throughout the study.
The treatment protocol consisted of 20 sessions of robotic gait training using the LEXO end-effector system. Each session was conducted on a one-on-one basis, with a single therapist working directly with each patient. As a personalized approach was taken to maximize the benefits of the training, the duration of each session ranged from 10 to 45 minutes, depending on the individual needs and progress of the patient. During the treatment, the assistance provided by the LEXO system was gradually adjusted to match the patient’s abilities; the aim was to maintain a walking rhythm that was both comfortable and demanding, progressively increasing the intensity as tolerated by the participant.
The assessment schedule included evaluations at three key time points: prior to the treatment (baseline), after 10 sessions of training, and after 20 sessions. The second evaluation, i.e. after 10 sessions, was performed to assess early changes in functional independence, walking endurance, and gait parameters. The final assessment, after 20 sessions, evaluated the cumulative effects of the treatment. All assessments were conducted using the same set of outcome measures to ensure consistency.
Data analysis
Statistical analysis was performed with SPSS23 software (Statistical Package for Social Sciences; SPSS Inc., Chicago, IL). Normality was checked before the statistical analysis; continuous data was represented as mean and standard deviation, and categorical data as percentages. The effect of the LEXO device on functional independence and gait was analysed using repeated measures ANOVA, with the Bonferroni correction for pairwise comparisons. A significance level of p < 0.05 was adopted.
RESULTS
Participant characteristics
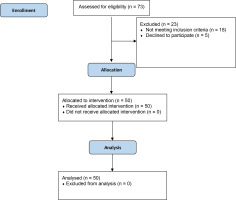
The study group included 50 patients, 37 of whom were men (74%), with no dropouts (Figure 2). The mean age was 55.76 ± 13.19 years. The demographic and clinical characteristics of the group are given in Table 1.
Table 1
Demographic and clinical characteristics of the patients (N = 50)
Performance
Main effect
The treatment achieved significant increases in functional independence, as noted by the FIM (F = 67.178, p = .000) and the BI (F = 47.035 p = .000), as well as in quality of life, indicated by the EQ-5D (F = 66.815, p = .000), and SIS (F = 53.599, p = .000).
For ambulation-related abilities, significant gains in walking capacity and fall risk were demonstrated, as measured with the 6MWT (F = 28.02, p = .000) and the TUG (F = 5.993, p = .018). Significant increases were noted for walking speed (F = 25.718, p = .000), cadence (F = 7.238, p = .010), and stride length (F = 7.809, p = .007).
Pairwise comparisons - baseline to 10 sessions
Significant differences (p < 0.001) for FIM, BI, EQ-5D, SIS, 6 MWT, TUG and walking speed were observed between baseline and the first evaluation, i.e. after 10 sessions. However, no significant differences in cadence or stride length were found (Table 2).
Table 2
Mean and significant differences at baseline, after 10 sessions, and after 20 sessions of training
| Mean ± SD Baseline | Mean ± SD 10 sessions | Mean ± SD 20 sessions | Δ Mean [95% CI] Baseline to 10 sessions | Δ Mean [95% CI] 10 to 20 sessions | Δ Mean [95% CI] Baseline to 20 sessions | |
|---|---|---|---|---|---|---|
| FIM | 95.69 ± 20.868 | 101.41 ± 18.422 | 108.76 ± 15.063 | 5.714* [7.950 to 3.479] | 7.347* [9.835 to 4.859] | 13.061* [17.015 to 9.108] |
| BI | 71.50 ± 21.882 | 76.20 ± 18.940 | 83.20 ± 16.835 | 4.700* [6.895 to 2.505] | 7.000* [9.674 to 4.326] | 11.700* [15.929 to 7.471] |
| EQ-5D | .258 ± .410 | .508 ± .315 | .668 ± .262 | .250* [.358 to .142] | .159* [.242 to .77] | .409* [.533 to .285] |
| SIS | 202.6 ± 37.134 | 210 ± 37.482 | 222.18 ± 38.868 | 7.740* [10.800 to 4.680] | 11.840* [15.833 to 7.847] | 19.580* [26.210 to 12.950] |
| 6MWT | 142.36 ± 110.139 | 158.90 ± 107.871 | 181.20 ± 115.713 | 16.540* [27.470 to 5.610] | 22.300* [33.006 to 11.594)] | 38.840* [57.035 to 20.642] |
| TUG | 27.235 ± 15.648 | 24.524 ± 15.154 | 23.361 ± 15.322 | 2.711* [.621 to 4.801] | 1,163* [.403 to 1.923] | 3.874* [1.257 to 6.491] |
| Walking speeda | 1.572 ± 1.157 | 1.819 ± 1.221 | 2.102 ± 1.275 | .247* [.403 to .091] | .283* [.457 to .109] | .529* [.788 to .270] |
| Cadencea | 68.914 ± 30.825 | 72.692 ± 32.052 | 79.268 ± 32.817 | 3.778 [10.780 to -3.224] | 6.575* [12.670 to .481] | 10.353* [19.893 to .813] |
| Stride lengtha | 60.670 ± 32.861 | 65.168 ± 34.045 | 70.371 ± 33.777 | 4.498 [11.584 to -2.588] | 5.204 [13.589 to -3.181] | 9.702* [18.308 to 1.095] |
Pairwise comparisons - 10 sessions to 20 sessions
Significant improvements (p < 0.001) in the FIM, BI, EQ-5D, SIS, 6 MWT, TUG, walking speed and cadence were found between 10 sessions and 20 sessions. No significant differences were found in stride length (Table 2).
Pairwise comparisons - baseline to 20 sessions
Significant improvements (p < 0.001) in FIM, BI, EQ-5D, SIS, 6 MWT, TUG, walking speed, cadence and stride length were found between baseline and after 20 sessions (Table 2).
DISCUSSION
LEXO is a new end-effector robotic system designed for the rehabilitation of patients with walking disabilities. However, its effect on walking disability in patients with CNS diseases remains unknown. Hence, this study aimed to describe the effects of training with the LEXO system on functional independence and gait parameters in people with central nervous system disorders.
After ten 10- to 45-minute sessions using the LEXO device, significant gains (p < 0.001) in functional independence and quality of life were observed, with greater improvements observed after 20 sessions. Improvements in functional independence have previously been reported using gait devices such as fixed exoskeletons34,35.
Our findings also indicate significant improvements (p < 0.001) in walking endurance after 10 and 20 sessions. This has been confirmed in previous studies, which found end-effector devices to improve walking endurance in stroke and spinal cord injury patients36–38. One study found 20 sessions of gait training with the Morning Walk end-effector system yielded a mean improvement of 52 meters in the 6MWT38. In contrast, a mean increase of 39 meters was observed in the present study after the same number of sessions; this difference could probably be attributed to the heterogeneity observed in our population, as both studies indicate significant differences in 6MWT between pre- and post-intervention.
Interestingly, previous studies have found robot-assisted gait devices to benefit heart rate and body fat percentage in patients with CNS disorders16,39. Hence, end effector systems could play a role in improving aerobic capacity; this plays an important part in improving functional independence and supporting recovery in patients with neurological diseases40,41. Significant reductions in fall risk were also noted after only 10 sessions. Similarly, in a study of robot-assisted end-effector-based gait training in patients with stroke, significant improvements were achieved in the TUG test after 10 sessions, which further improved after 20 sessions42.
In the present study, all gait parameters (walking speed, cadence, and stride length) significantly improved (p < 0.001) after 20 sessions using the end-effector device. However, after 10 sessions, improvements were noted in walking speed, but not in cadence or stride length. In contrast, Geroin and colleagues observed improvements in all three outcomes after only 10 sessions of 50 minutes43. However, robot-assisted gait training (RAGT) systems are more effective in improving gait speed and functional outcomes other than spatiotemporal or kinematic parameters, and it is possible that more intense training might be required to enhance the latter44.
The positive results achieved with the LEXO device could be attributed to a combination of several factors. For example, it is possible that the continuous feedback provided by its virtual reality system may improve adherence to therapy45,46. Gait assistive devices might allow a greater number of movement repetitions and avoid possible disuse of the lower extremities47.
Our findings are in line with a recent Cochrane review, which concluded that using electromechanical-assisted gait training devices can improve independent walking in the rehabilitation of stroke patients. It also showed that end-effectors might present several advantages over the use of exoskeletons for stroke rehabilitation since the use of an end-effector system showed better results for both gait speed and distance achieved48. This advantage may stem from the that fact that end-effectors provide less assistance to the patient than exoskeletons, as they do not support the knee and hip and the patient is responsible for generating active movement49. According to motor learning theories, this could lead to a more complex motor task challenge, allowing the patient to be closer to the optimal learning point for the motor task50. Additionally, by reducing the assistance provided by the device, the patient bears a greater metabolic cost during gait, enhancing the neuroplastic changes induced by the exercise51,52.
No severe safety-related incidents were experienced while using the LEXO system, suggesting it is safe for use in clinical settings for treating various CNS movement pathologies. While the LEXO system appears at least equal to other RAGT end-effector systems such as morning walk or G-EO17,53,54; however, more studies comparing different devices in the same context are needed to confirm this.
Limitations
The study has some limitations may limit the generalizability of our findings. Most importantly, all participants were recruited in Madrid, the sample was quite small, and no control group was included. Furthermore, as the sample was quite heterogeneous, with a predominance of patients with brain injuries and under-representation of other CNS pathologies, care should be taken when generalizing our observations to other CNS pathologies. Finally, as daily activities were not assessed using performance observation scales, the data has a significant subjective component.
CONCLUSIONS
The LEXO is an end-effector robotic system that allows intense gait training within a non-inversive virtual reality and could decrease the therapist workload in therapy sessions.
The device appears safe for use in clinical settings for a variety of people with CNS movement disorders. Training with the LEXO device can help improve functional independence, quality of life, endurance, and certain gait parameters (walking speed, cadence, and stride length), and decrease the risk of falls in people with CNS disorders. Although these results need further validation through additional research studies, the potential of LEXO to improve functional gait in patients with CNS disorders appears promising. Additionally, future studies should aim to classify participants by pathology and/or severity to identify the clinical characteristics of those who may benefit more from the device.
Our findings provide data regarding the use of the end-effector system for gait rehabilitation in patients in the Spanish population who present difficulties in ambulation due to CNS alterations.