Introduction
In recent years, there has been a significant improvement in knowledge about facial fat compartments and their age-related changes. Subcutaneous fat was once believed to be a single uniform mass. However, recent studies indicate that this fat is divided into multiple compartments separated by a facial retaining system composed of fibrous fascial ligaments [1–4]. In general, fat pads can be categorized into superficial and deep compartments. In the midface region, the first group includes the nasolabial fat, the infraorbital fat, and the superficial medial cheek fat. The deep compartments, situated below the superficial musculoaponeurotic system (SMAS), include the sub-orbicularis oculi, buccal fat pad, deep lateral cheek pad, and deep medial cheek pad [1, 2, 5–8].
Findings from clinical observations and studies clearly indicate that various fat compartments undergo various age-related changes, with volume deflation being the main process. In addition, an inferior displacement along with increased distances between the fat pads is observed [1, 2, 7–9]. Volume loss does not have a global character, but is rather limited to the deep fat compartments supporting the overlying structures [1, 3, 8, 9]. Some studies have even demonstrated that some superficial facial fat seems to undergo hypertrophy in the elderly [10, 11]. The midfacial “pseudoptosis” theory suggests that selective atrophy of the deep medial cheek fat compartment (DMCFC) is the main factor contributing to a loss of anterior projection of nasolabial and nasojugal folds (sagging of the superficial compartments), inferior displacement of the lower lid (V-deformity) and unpleasant aesthetics of the palpebromalar groove, nasojugal fold, and tear trough [1, 7–12].
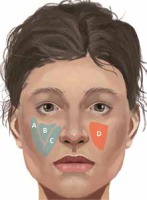
The increased knowledge of fat pads’ anatomy and their age-related changes have altered the modern facial rejuvenation strategies, previously based on the lifting of the SMAS and skin along with fat compartments as one mass [1, 2, 8, 9, 13]. Nowadays, one can observe a trend of a shift towards specific compartment augmentation with a fat graft or filler as a primary procedure of choice for anterior midfacial projection restoration and improvement of pseudoptosis symptoms [2, 3, 7, 8, 12–14]. In this concept, DMCFC is considered to be the most important structure which should be addressed in the first place (Figure 1) [8, 13].
Figure 1
Superficial (blue color) and deep (red color) fat compartments crucial for midface region rejuvenation. A – Superficial lateral cheek fat compartment, B – superficial middle cheek fat compartment, C – superficial nasolabial fat compartment, D – deep medial cheek fat compartment
Regardless of multiple investigations on understanding facial physiologic age-related changes, there still is much to be done in terms of fat compartments. The formulated aging mechanism of those structures is mainly based on clinical observations and cadaveric dissections or imaging studies such as computed tomography (CT) or magnetic resonance imaging (MRI). Therefore, it should be considered a conjectural concept rather than a straightforward conclusion supported by quantitative data [8–10, 12]. Little is known about the internal structural characteristics of fat compartments and their differences among various facial regions and age groups. Such information could play a major role not only in better understanding of the aging process but also in facial rejuvenation results analysis and formulation of new patient-specific treatment algorithms. Some previous studies demonstrated that the age-related changes within soft tissues of the face can be documented by means of imaging studies such as MRI or CT [15, 16], but those methods are not widely available and generate substantial costs.
In our opinion, valuable structural characteristics of the facial fat compartments may be accurately determined with a less expensive and more accessible method, ultrasonographic shear wave elastography (SWE).
Aim
Our study aim was verification of the potential of this technology in the quantitative tissue elasticity assessment of facial fat, taking the example of the deep medial cheek fat pad, due to its major role in pseudoptosis etiology. Furthermore, we determined the age-specific reference values for DMCFC elasticity. Finally, the relationship of DMCFC elasticity with body mass index (BMI) and DMCFC thickness was analyzed.
Material and methods
The study, conducted at the University Department of Plastic Surgery and at a private plastic surgery and esthetic medicine clinic in Warsaw, included all consecutive female patients qualified for high-intensity focused ultrasound facial treatment. Only patients aged at least 18 years at the time of enrollment were qualified for the study. Women with visible scars or other skin lesions on the face, present or past history of connective tissue diseases, other autoimmune disorders, diseases of the skin and subcutaneous tissue and/or peripheral blood vessels, allergy or atopy, surgery or trauma involving the face, as well as active current and past smokers, were non-eligible for the study.
The study procedures were carried out in accordance with the Declaration of Helsinki. The protocol of the study was approved by the Institutional Bioethical Committee, and written informed consent was sought from all the subjects.
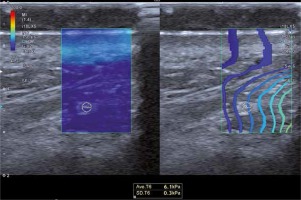
Prior to the laser treatment, all participants were subjected to ultrasonographic examination and elastography of the DMCFC. The examination was carried out with the patient in a supine position. Sonographic scans were obtained with a Toshiba iAplio 900 ultrasonograph with a 5–18 MHz transducer (Canon Medical Systems, Nasushiobara, Japan). During the examination, the face was covered with a hydrogel pad and a thick layer of gel. The transducer was placed perpendicularly to the skin, and transverse scans were obtained. Upon visualization of the area of interest, the thickness of the DMCFC in millimeters was measured. Then, SWE was performed, after stabilizing the elastographic image. The region of interest (ROI) was placed in the center of the screen, to cover approximately 80% of the examined structure. Three measurements were taken for each ROI and the average result was recorded. The reference value for the elasticity modulus was set at 100 kPa. Representative elastographic images are presented in Figure 2.
Statistical analysis
Normal distribution of the study variables was verified with the Shapiro-Wilk test, and their statistical characteristics were presented as arithmetic means and their standard deviations (SDs), as well as medians and ranges. Power and direction of relationships between pairs of the variables were estimated on the basis of Spearman coefficients of rank correlation (R). The effects of multiple predictors were analyzed using multiple regression analysis; the regression coefficient (β) was calculated, along with its standard error (SE) and coefficient of determination for the regression model (R2). Intergroup comparisons were conducted with the Kruskal-Wallis test with Dunn post-hoc tests. Reference ranges for elastographic parameters were defined with two methods, as ± 2 SD and on the basis of ROC analysis. During the latter, cut-off values of the elastographic parameters which optimally distinguished between various age categories were identified, along with their sensitivity, specificity, positive and negative predictive values (PPV and NPV, respectively) and the areas under ROC curves (AUC) with their 95% confidence intervals (95% CIs). All calculations were carried out with the Statistica 10 package (StatSoft, United States) with the threshold of statistical significance set at p ≤ 0.05.
Results
Characteristics of study participants
The study included 89 women aged between 18 and 63 years (mean: 45.9 ±14.2 years). The study group was divided into four age categories: women aged 39 years or younger (n = 18, 20%), 40- to 49 years old (n = 28, 31%), 50 to 59 year old (n = 29, 33%), and 60 years old or older (n = 14, 16%). Descriptive statistics for the thickness and elasticity of the DMCFC, as well as other characteristics of the study participants, are presented in Table 1.
Factors determining strain of the deep medial cheek fat compartment
A strong significant inverse correlation was found between age of the study participants and DMCFC elasticity (R = –0.838, p < 0.001). Moreover, age showed a moderately strong correlation with DMCFC thickness (R = 0.254, p = 0.001). DMCFC elasticity correlated also inversely with BMI of the examined women (R = –0.258, p = 0.001). No statistically significant correlation was observed between BMI and DMCFC thickness (R = 0.103, p = 0.171). Finally, a significant inverse correlation was documented between DMCFC thickness and elasticity (R = –0.292, p < 0.001). However, multiple linear regression analysis demonstrated that the age of the study participants was the only independent determinant of DMCFC elasticity among the three variables mentioned above (age, BMI, and DMCFC thickness) (Table 2).
Table 2
Results of multiple linear regression analysis, documenting the effects of age, BMI and DMCFC thickness on DMCFC elasticity
| Predictor | DMC (R2 = 0.754) | ||
|---|---|---|---|
| β | SE | P-value | |
| Age [years] | –0.837 | 0.040 | < 0.001 |
| BMI [kg/m2] | –0.050 | 0.039 | 0.197 |
| DMCFC thickness [mm] | –0.058 | 0.039 | 0.144 |
During the next stage, we compared the elasticity of the DMCFC in women from various age categories. Participants from all age categories except from women aged 50–59 years and older than 60 years differed significantly in terms of DMCFC elasticity (Table 3).
Table 3
Comparison of DMCFC elasticity (in kPa) in participants from various age categories
Reference values for the deep medial cheek fat compartment strain
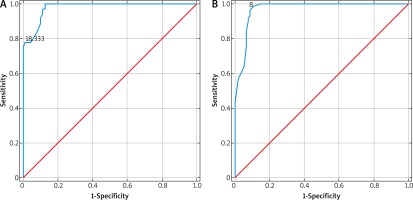
Considering the results presented above, we determined the reference values for DMCFC elasticity for three age categories: under 40 years, 41–49 years, and above 50 years. The cut-off values for DMCFC elasticity estimated during ROC analysis provided excellent accuracy in distinguishing between women from various age categories (Figure 3). Furthermore, the reference values determined with this method to a large degree overlapped with the reference intervals defined as ± 2 SD for a given age category (Table 4).
Discussion
Elastography, a sonographic technique to estimate tissue elasticity, was first implemented in clinical practice in the 1990s [17]. Elastography measures the deformability of tissues caused by an external force, typically compression with an ultrasonographic transducer (strain elastography – SE), or the velocity of shear wave propagation within the tissue (shear wave elastography – SWE) [18, 19]. Both literature data and our experience indicate that SWE, measuring tissue elasticity in kPa, is a more accurate and objective elastographic technique than SE [19, 20]. Previous studies confirmed the applicability of elastography to many medical disciplines, primarily as a method to distinguish between normal and pathologically altered tissues [21]. However, a growing body of evidence suggests that elastography may also find application in esthetic medicine, as an instrument to examine the elasticity of the skin and adjacent structures [22–24].
The DMCFC lies between the periosteum of the maxilla and deep muscles, and therefore is under constant pressure, which may be one of the factors responsible for its relatively large atrophy with age. Due to its crucial role in age-related facial contour incongruity and importance in modern rejuvenation algorithms, we found it to be a perfect target for our preliminary study utilizing SWE technology.
Analysis of the results clearly showed that aging was associated with a statistically significant decrease in DMCFC elasticity; moreover, age turned out to be the only independent determinant of deep medial cheek fat compartment stiffness in the study group. To the best of our knowledge, there is no published research analyzing the elastographically determined elasticity of any facial fat compartment and its age-related changes; thus, we can only speculate about potential causes of this phenomenon.
Probably, a key to the age-related decrease in DMCFC elasticity might be its microscopic structure and changes it undergoes with age. In line with the microscopic classification of subcutaneous fat, proposed by Ghassemi et al. [25], it represents a type 1 fat tissue with a loose linkage between the collagenous meshwork surrounding the adipocytes, the facial muscles, and the periosteum. The extracellular matrix of white adipose tissue, although sparse, plays an important active role in DMCFC morphology and physiologic processes [26]. Therefore, the primary cause of the hereby documented age-related decrease of the elasticity values of the fat pad might be degradation of the otherwise sparse collagen network in this tissue [27]. A similar age-progressive process, mediated by matrix metalloproteinases released from dermal keratinocytes, has already been documented in the case of type I collagen, the major structural protein of the skin [28]. Recent evidence suggests that aside from age, the activity of matrix metalloproteinases may also be stimulated by increased subcutaneous white adipose tissue [29, 30]. However, unlike the amount of subcutaneous fat, the volume of the deep fat compartments does not seem to be BMI-dependent [31]; this was also confirmed in our study, which did not demonstrate a significant correlation between DMCFC thickness and BMI of the study participants. Nevertheless, we observed an inverse correlation between BMI and DMCFC elasticity, which suggests that an increase in body weight of the study participants might contribute to local or systemic changes in fat ultrastructure, like the mentioned activation of metalloproteinase activity and resultant degradation of the collagen network.
Taking into account the statistically significant correlations between participant age and DMCFC elasticity, we have defined its reference values for three age categories: < 40 years, 40 to 49 years, and 50 years and older. The appropriateness of such stratification was verified based on the intergroup comparisons of the elastographic parameters. The reference values were estimated with two methods, as ±2 SD and using ROC analysis. Importantly, both sets of reference values overlapped to a large degree, and the cut-off values of DMCFC elasticity estimated during ROC analysis provided excellent accuracy in distinguishing between women from various age categories. The hereby presented reference values may find application in esthetic medicine, e.g. to determine whether the DMCFC in a given patient is tight enough to be suitable for a specific rejuvenation procedure or to objectively evaluate the treatment results.
Overall, our study confirmed that SWE can be used to assess the elasticity of the facial fat compartment in a reliable, quantitative manner. This technology may be a valuable addition to the facial aging process research armamentarium. The elasticity of the fat pad might be a hallmark of metabolic and structural properties of adipose tissue and its extracellular matrix. Further studies, utilizing fat histological and volumetric analysis, are needed in order to confirm and fully understand this relation. Moreover, SWE might have clinical application in routine aesthetic medicine practice. Ability to evaluate fat compartments’ structural characteristics enables objective analysis of facial rejuvenation results, eventually leading to improvements of treatment algorithms. Rapid assessment of patient fat compartments’ strain value combined with the creation of a reference database of tissue elasticity of each significant fat compartment in a certain age group would allow treatment option personification, tailored to the specific need.
Our study was not free from potential limitations. The first of them was a relatively small patient sample size, especially in the context of age group analysis. Nevertheless, to the best of our knowledge, this was the only elastographic study of the facial fat pad that has been conducted thus far. Second, it cannot be excluded that the elastographic parameters determined in this study were influenced by some non-identified confounders. We tried to overcome this potential bias, enrolling solely patients who satisfied the most exhaustive list of inclusion and exclusion criteria. Finally, the hereby presented findings might be biased due to some drawbacks inherent to elastographic examination of a relatively thin layer of examined fat pad, located in close proximity to other tissues with higher values of Young’s modulus, such as muscles and bones, or due to subjective selection of the ROI. We did our best to neutralize these potential limitations, using a high-frequency transducer and a hydrogel pad, reducing the diameter of the ROI and repeating each measurement in pursuit of better reproducibility. Finally, our work due to its preliminary character determined the elasticity reference values for only DMCFC. Future research will cover all significant superficial and deep facial fat compartments, providing an overall insight into tissue elasticity distribution among various facial regions, age groups and sexes.
Conclusions
Shear-wave elastography enables quantitative evaluation of facial fat pad elasticity, creating a new frontier of research into age-related processes. Our study results show that elastographically determined elasticity of the DMCFC decreases significantly with age. The elasticity of the fat pad might be an indirect indicator of metabolic and structural properties of facial adipose tissue and its extracellular matrix.