Summary
The rate of atherectomy utilization in peripheral artery diseases growing. The two atherectomy devices available on the market and used most frequently are the directional and rotational ones. The aim of the study is to compare the long-term outcomes after peripheral artery diseases (PAD) revascularization with directional and rotational atherectomies. This was a single-center, retrospective study of obstructive and symptomatic PAD patients who underwent revascularization with atherectomy. There were no differences between groups in bailout stenting, amputations or death rate. Target lesion revascularization (TLR) was more frequent in the directional atherectomy group. In this hypothesis-generating study of patients with symptomatic PAD, revascularization with rotational atherectomy had a lower rate of TLR when compared to the directional one.
Introduction
The rate of the peripheral arterial disease (PAD) has been constantly growing. Four percent of the adult population in the US will develop claudication in middle age and approximately 14% after the age of 70 [1, 2]. Simultaneously, new technologies for PAD treatment have emerged, including pharmacological regimens, endovascular and open surgery and stem cell therapy [3]. Nevertheless, the most frequent treatment for severe claudication and critical limb ischemia remains endovascular procedures and one of the most intensively developed technologies is atherectomy [4]. The main difference between the two devices compared in this study is the plaque modification mechanism. In rotational atherectomy the cutting blades are placed on the tip of the device, whereas in the directional one the cutting blades are placed on the side of the catheter. The asymmetrical rotational crown coated with microdiamonds is used in orbital atherectomy. Some devices use ultrasound waves for plaque modifications [4]. Novel devices are equipped with an intravascular imaging system such as optical coherence tomography (OCT) [5] or they use laser light to debulk the lesion [6]. The two atherectomy devices available on the market and used most frequently are the directional and rotational ones. Nonetheless, there is a lack of direct comparison between these two types of atherectomy in PAD.
Aim
Therefore, the aim of the study was to compare long-term outcomes after PAD endovascular revascularization performed with two types of atherectomy devices – rotational (AR) (Phoenix Philips) and directional (AD) (SilverHawk Medtronic).
Material and methods
Subjects
The paper is based on a retrospective study of 182 consecutive patients with symptomatic PAD who underwent endovascular revascularization with directional (AD) or rotational atherectomy (AR) between 2010 and 2015 at the San Antonio Endovascular & Heart Institute. Adult patients (> 18 years old) with both intermittent claudication (Rutherford 3rd class) and critical limb ischemia (CLI) (Rutherford 4th–6th class) were included if they had at least 1 lesion with > 70% diameter stenosis confirmed on the live quantitative vessel angiography in a lower extremity artery. Patients with in-stent restenosis were excluded.
Procedural characteristic and pharmacological regimen
Directional (Silver Hawk, Medtronic) and rotational (Phoenix Philips) atherectomy (AT: atherectomy) devices were applied in this study. The choice of the AT type was left to the operator’s discretion. Atherectomy was followed by low-pressure balloon post-dilatation if residual stenosis was > 30%. A distal protection system was not used for any patients. Angiographic success was defined as post-procedural TIMI 3 flow, no dissection and residual stenosis < 30%. If angiographic success was not achieved, bail-out stenting was performed. Aspirin (81 mg/day) was continued indefinitely whereas clopidogrel (75 mg/day) was advised to be continued for 12 months after the procedure, as well as atorvastatin at the maximum tolerable dose, usually 40 mg daily.
Atherectomy devices
The Silver Hawk (Medtronic) plaque excision system is a forward cutting directional atherectomy device. This can be used with or without concurrent percutaneous balloon angioplasty and stenting. The device consists of a rotating blade inside a tubular housing with a collection area nosecone. This catheter is connected to a battery-driven motor which spins the cutter.
The device comes in various sizes to enable atherectomy in vessels with diameters of 1.5–7.0 mm. The advantage of atherectomy performed with the Silver Hawk device is the directional control, which makes it easier to remove eccentric lesions. As the device is advanced through the lesion, plaque is excised and packed in the nosecone. Different planes of excision are achieved by rotation of the device. Distal embolization remains a major disadvantage with these systems and hence the use of embolic protection devices is recommended in large and heavily calcified vessels [7].
The Phoenix Atherectomy System has a minimum working length of 130 cm. The inward-cutting helical blade sits within a housing that acts as a shield and has an open area to expose the cutter. The cutter is rotated at high speeds (10,000 to 12,000 rpm) and shaves material directly into the housing. The debulked material is then conveyed to the proximal part of the catheter by an Archimedes screw on the outside of the torque shaft, which continuously conveys the excised plaque through the handle and into a collection reservoir outside the patient. The tip of the largest available catheter (2.4 mm) can be deflected to various degrees and rotated so that the cutter can debulk eccentrically to a larger diameter than the catheter itself. The controls for deflection and rotation are housed in the catheter handle with a self-contained battery-powered motor, and the operation of the Phoenix does not require any capital equipment components. This atherectomy can be used in vessels with diameter ranging from 2.0 to 7.0 mm.
Both devices are approved for use in atherectomy of the peripheral vasculature and are not approved for use in coronary, carotid, iliac, or renal arteries [8].
Study endpoints and definitions
Because of the observational nature of this study, no preliminary hypothesis was generated. Target lesion revascularization (TLR) was considered the primary endpoint and was defined as any symptom-driven revascularization within a previously treated segment. Unplanned amputation related to a previously treated vessel, death and change in Rutherford class were regarded as secondary endpoints. Moreover, incidents of vessel perforation, dissection, distal embolization, and bailout stenting were collected.
Safety and ethics
This retrospective study was conducted in accordance with standard ethics guidelines. Endovascular procedures were carried out by experienced interventional cardiovascular teams in a high-volume center with a vascular surgery back-up within 30 min of transportation.
Owing to the observational and retrospective nature of this study, neither the patients’ consent nor ethics committee approval was required.
Data collection and follow-up
Clinical and procedural data were collected on case report forms generated by the hospital electronic system, containing all patient hospitalization and discharge information. This system is audited for institutional quality assurance by private insurance companies and the state health fund.
The long-term follow-up data were collected during ambulatory check-ups or by telephone. The follow-up office visits were usually scheduled each 3–5 months. Some patients had phone visits due to lack of symptoms; nevertheless an office-based follow-up was scheduled on a later date. All outcomes of interest were confirmed using hospital discharge charts. Three patients met exclusion criteria for in-stent restenosis and one patient was lost to follow-up.
Statistical analysis
Continuous variables are presented as mean ± SD or median (IQR). Data were compared using the t-test for parametric or Mann-Whitney U-test for non-parametric continuous variables. Categorical variables are reported as frequencies (percentages) and were compared using the Fisher exact test. Survival curves were constructed using the Kaplan-Meier estimates and were compared using the log-rank test. All reported p-values are 2-tailed, and p < 0.05 was considered significant. GraphPad 6 Prism was used for the statistical analysis.
Results
The baseline characteristic of both groups is shown in Table I. There were no significant differences between the groups in baseline demographics except a higher rate of critical limb ischemia in the AR group. The mean follow-up for AD and AR was 282.6 ±147.4 and 255.7 ±186.1 days, respectively (p = 0.44).
Table I
Demographic characteristics
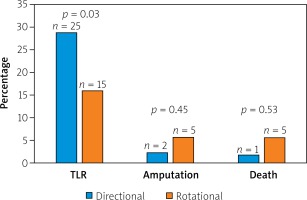
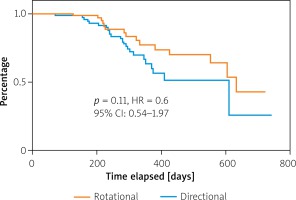
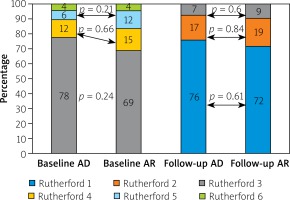
Lesions characteristics were similar between groups with the mean number of lesions equaling 1.5 and 1.4 per AR and AD patient, respectively. In both groups the primary lesion location was the anterior tibial artery. Furthermore, there were no significant differences between the groups in terms of lesion morphology in the TransAtlantic Inter-Society Consensus (TASC) and the number of chronic total occlusion. The procedural characteristics are shown in Table II. The number of periprocedural complications was low and similar between groups (Table III). The TLR was significantly more frequent in the AD group than in AR (AD: 25 (29.0%) vs. AR: 15 (15.9%) p = 0.03) (Figure 1). There were no significant differences in the rate of death (AD: 1 (1.7%) vs. AR: 5 (5.7%); p = 0.54) or amputations (AD: 2 (2.3%) vs. AR: 5 (5.7%); p = 0.45). There were no significant differences in time to TLR (Figure 2), amputation or death rate between the groups. There were no significant differences in the Rutherford class between the groups during follow-up. However, comparing before and after revascularization assessment there was a significant drop in the Rutherford class in both groups (< 0.0001), as shown in Figure 3.
Table II
Procedural characteristics
Discussion
This study presents the single center outcomes of patients revascularized with the two types of atherectomy: directional and rotational. According to the available literature, the present study describes for the first time long-term outcomes of revascularization with two types of atherectomy in claudication and critical limb ischemia patients. The demographics were comparable between the groups with the exception of the CLI rate, being in favor of the AD group. Nevertheless, the procedural characteristics show no differences between the groups. The periprocedural complications, death and amputation were comparable between the groups. However, the TLR rate differed significantly in favor of the AR group.
To the best of our knowledge this is the first comparison of the two types of atherectomy used in PAD revascularization. In the EASE study the outcomes of PAD treatment with the Phenix Philips atherectomy device were comparable to the results we achieved. The rate of periprocedural complications was low and comparable (perforation – 5%, dissection – 2%, distal embolization – 1%). However, in the 6-month follow-up the TLR rate was 12.4% and patients with CLI were excluded from the study [8]. The second rotational atherectomy device available on the market is the Pathway Jetstream Boston Scientific. In the published studies the periprocedural complications were more frequent than in our study. The authors reported 9% of dissections and 10% of minor embolization. The perforation scale was similar. Total rate of TLR after 12 months of follow-up was 27.1%, which is higher than in our study [9]. In the other study the outcomes of PAD treatment with the Pathway Jetstream Boston Scientific were compared between patients with and without diabetes mellitus (DM). The TLR rates after 12 months of follow-up were 20% and 28% in DM and non-DM patients, respectively [10]. There is an abundance of reports on the PAD treatment with directional atherectomy. In the TALON study, SilverHawk was used to treat PAD in 601 patients. The rate of TRL after a 6- and 12-month follow-up was 10% and 20%, respectively [11]. In the LA-DEFINITIVE study, where 800 subjects with both claudication and CLI were revascularized with directional atherectomy (SilverHawk/TurboHawk Medtronic), the primary patency rate was 81.5% [12].
The unification of indications, contraindications, atherectomy device choice and technique of debulking procedure, according to peer-reviewed expert opinion based on trials and registries could improve outcomes after revascularization with atherectomy in PAD, as has already happened in the coronary field [13].
A novel technology that may by combined with atherectomy in PAD is local drug delivery after revascularization. Early reports on the combination of plaque modification with atherectomy and subsequent DEB seem to be promising [14, 15]. Nevertheless, a meta-analysis published recently by Katsanos et al. showed that the relative risk of all-cause mortality increases after the use of paclitaxel-coated device in PAD treatment in 2 and 5 years by 68% and 93%, respectively [16]. Novel technologies, including local drug delivery nanotechnology, may soon become available for the follow-up treatment of plaque modifications after atherectomy [17]. Another interesting technology is directional atherectomy combined with optical coherence tomography, which allows more efficient debulking of the lesion while sparing adventitia. This technology is used in the Pantheris catheter, the safety and efficiency of which were confirmed in the Vision study [5].
To summarize, this study shows that the outcomes of the application of the rotational atherectomy in PAD are better than in the case of the directional device. This fact generates a hypothesis that AD could injure the vessel wall more deeply than AR. Nonetheless, it should be confirmed in future studies where the AT revascularization is followed by intravascular imaging techniques, such as optical coherence tomography (OCT) or intravascular ultrasound (IVUS). Despite encouraging results of lower extremities’ artery revascularization with atherectomy, still plain old balloon angioplasty and stenting is recommended for invasive treatment [18, 19]. Moreover, laser ablation is no better than mechanical debulking with atherectomy. The only confirmed recommendation for endovascular laser ablation is in stent restenosis [20].
The main drawbacks of this analysis are those inherent to any single-center observational study [21] along with the difference of the CLI rate between the groups. The exact data on the very long below-the-knee chronic total occlusion are unavailable. The ankle brachial index (ABI), ultrasonography doppler and toe pressure were not performed at each visit, making these data unsuitable for statistical analysis. During the study only one type of atherectomy device was available in the study site at the same time. This study is hypothesis generating only.
Conclusions
In this hypothesis-generating study of patients with symptomatic PAD, revascularization with the rotational atherectomy had a lower rate of TLR when compared to the directional one, despite the increased CLI rate in the AR group. The periprocedural complications, amputations and death rate were comparable. Nevertheless, this should be confirmed in further controlled randomized trials.