Introduction
Acne vulgaris is a common chronic skin disorder of the sebaceous follicles, which can affect up to 50.9% of women and 42.5% of men throughout their 20s and may continue to occur throughout adulthood [1]. The disease pathogenesis is complex and involves interplays between hyperplasia of the sebaceous glands, the subsequent formation of microcomedones associated with hyperkeratinisation of the follicular wall, and the induction of inflammatory reactions in keratinocytes and sebocytes [2, 3]. Apart from Cutibacterium acnes, which can perpetuate the pathogenetic process of acne through the induction of proinflammatory and chemotactic molecules [4, 5], acneic skin is frequently colonized with Staphylococcus aureus. Using a high-throughput sequencing approach, Dreno et al. [6] found that Staphylococci were more abundant on the surface of comedones, papules, and pustules than on non-lesional skin. Taheri et al. [7] also reported that light emitted from digital screens may promote the proliferation of S. aureus, which may in turn be associated with acne pathogenesis. Jusuf et al. [8] were able to identify S. aureus growth in both non-inflammatory and inflammatory acne lesions. Additionally, both antibiotics and dietary supplements containing probiotics may reduce the S. aureus carriage rate in facial acneic skin [9, 10].
Bacteriocins are bacterially produced, ribosomally synthesized antibacterial peptides secreted for defence against the growth of closely related bacterial species [11, 12]. Bacteriocins produced by Gram-positive, aerobic, and endospore-forming Bacillus subtilis – including subtilin and subtilosin – are active against many strains of gram-positive bacteria, including S. aureus [13]. They mainly restrict the growth of pathogenic bacteria by promoting pore formation on the target cell surface. Given their safety and stability [12], we reasoned that topically applied bacteriocins from B. subtilis could have therapeutic potential in acne by promoting S. aureus decolonization.
Aim
Two distinct investigations were therefore undertaken. First, we conducted a 60-day pilot study on the effect of topically applied bacteriocins from B. subtilis on the absolute abundance of S. aureus in the skin of 12 patients with mild-to-moderate acne. Second, we designed an 8-week, uncontrolled, open-label, multicentre clinical study to investigate whether the topical application of bacteriocins from B. subtilis may reduce the number of inflammatory and non-inflammatory lesions, as well as Global Acne Grading Scale (GAGS) scores, in patients with mild-to-moderate acne.
Material and methods
Materials
The topical cream tested in this study contained bacteriocins from B. subtilis (1% weight/weight; Biodue S.p.A., Tavarnelle Val Di Pesa, Italy) in a moisturizer base.
Procedures
Patients with active facial acne of mild-to-moderate severity (Global Acne Grading Scale [GAGS] score from 1 to 30) [14, 15] were eligible for inclusion. Subjects were excluded if they had previously received oral retinoids, oral antibiotics, tretinoin, or benzoyl peroxide. In addition, patients with diabetes mellitus, endocrine disease, or severe physical illnesses and those who were currently using oral contraceptives, implantable contraceptives, or steroids were excluded [16]. All participants involved in the microbiological and clinical investigations were asked to withdraw any topical product 14 days before the beginning of the studies. Moreover, they were not allowed to use any topical intervention throughout the entire study period. The study participants were instructed to apply the topical cream over acne areas twice per day (morning and evening) for 60 days in the microbiological study and for 8 weeks in the clinical study. There were no known protocol deviations during the study. Both investigations were approved by the local Ethics Committee (identifier: 2021/14E) and were in accordance with the tenets of the Helsinki Declaration. Written informed consent was obtained from all participants.
Microbiological study
The microbiological study was aimed at assessing whether topical application of the cream containing bacteriocins from B. subtilis was able to promote S. aureus decolonization in acne areas. A total of 12 patients (6 males and 6 females; age range: 22–35 years) were recruited from Italian private practices. The sample size for this pilot study was chosen based on feasibility and costs. Skin swab specimens from facial acne areas before and after 60 days of topical treatment (paired samples) were obtained by trained personnel. Quantitative real-time PCR for absolute S. aureus quantitation was carried out as previously described [17]. In brief, total nucleic acid from acneic skin swab specimens was amplified and the DNA concentration was quantified using the CFX96 Real-Time System (Bio-Rad, Hercules, CA, USA). Standard curves for the absolute abundance values of S. aureus in collected specimens were constructed using seven 10-fold dilutions (from 4 ng/µl to 4 fg/µl) of S. aureus USA300 [18].
Clinical study
This 8-week, uncontrolled clinical study had an open-label design. We did not conduct a power analysis to determine sample size; instead, we used a convenience sample. A total of 373 Caucasian patients with mild-to-moderate acne (139 males and 234 females, mean age: 22.0 ±6.4 years) were included. All participants were enrolled from Italian private practices and dermatology clinics. Clinical assessments were performed at baseline and 8 weeks thereafter. The primary endpoint of the clinical study was the changes in the number of inflammatory and non-inflammatory lesions, as well as GAGS from baseline to the end of the study. GAGS considers 6 locations on the face and chest/upper back, with a factor for each location based roughly on surface area, distribution, and density of pilosebaceous units [14]. The severity was graded as mild if the score was 1–18, moderate with scores from 19 to 30, severe with scores from 31 to 38, and very severe with scores of more than 38 [15]. The secondary endpoints were tolerance and overall satisfaction with the topical cream. Tolerance was assessed by asking patients about any signs or symptoms of local (burning, itching, or stinging sensation) or systemic adverse reactions. The overall satisfaction was rated on a 4-point scale, as follows: excellent, good, average, or poor.
Statistical analysis
Data are presented using descriptive statistics. Paired Student’s t-tests were used to compare pre- and post-treatment data in both investigations. All analyses were carried out in SPSS (version 20.0; IBM, Armonk, NY, USA), and statistical significance was determined by a 2-tailed p-value < 0.05.
Results
Microbiological study
The results of the pilot microbiological study revealed that the absolute abundance of S. aureus in facial acne areas in the 12 subjects who underwent paired skin swab sampling decreased after 60 days of treatment with topically applied bacteriocins from B. subtilis. Based on quantitative real-time PCR results, the mean absolute abundance of S. aureus was 2024 ±338 fg/µl at baseline and 1254 ±214 fg/µl at 6 months, i.e. a 38% decrease (p < 0.001).
Clinical study
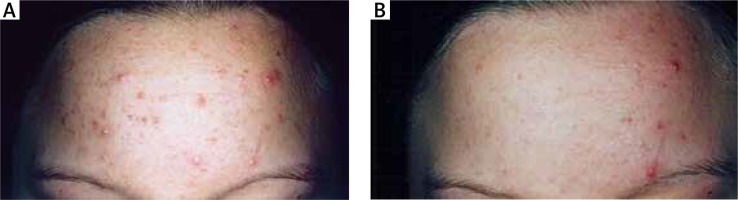
All patients (n = 373) successfully completed the study. The number of inflammatory lesions significantly decreased by 59% (p < 0.001, paired Student’s t-test) after 8 weeks of treatment with the topical cream; the number of inflammatory lesions was 10.89 ±8.61 at baseline and 4.39 ±5.92 at 8 weeks. Similarly, the number of non-inflammatory lesions significantly decreased by 58% (p < 0.001, paired Student’s t-test) after 8 weeks of treatment with the topical cream; the number of non-inflammatory lesions was 13.36 ±8.60 at baseline and 5.60 ±4.39 at 8 weeks. The mean GAGS score was 11.98 ±9.21 at baseline and 5.17 ±4.22 at 8 weeks, i.e. a 56% decrease (p < 0.001, paired Student’s t-test). Representative images of a patient before (left panel) and after (right panel) 8 weeks of treatment with bacteriocins from B. subtilis are shown in Figure 1. The treatment was well-tolerated, and none of the patients reported burning, itching, or stinging sensation after topical application. No patient discontinued treatment due to adverse local or systemic effects. The overall satisfaction with the topical cream was rated as excellent by 188 (50.4%) patients, good by 168 (45%) patients, average by 15 (4%) patients, and poor by 2 (0.6%) patients.
Figure 1
Representative images of a patient before (A) and after (B) 8 weeks of treatment with bacteriocins from Bacillus subtilis. A significant improvement in the clinical appearance of acne was evident (baseline: number of inflammatory lesions = 14; number of non-inflammatory lesions = 19; after 8 weeks: number of inflammatory lesions = 3; number of non-inflammatory lesions = 7)
Discussion
The results of our microbiological and clinical studies indicate that topically applied bacteriocins from B. subtilis - a safe bacterial-derived ingredient [13] – may decrease the number of both inflammatory and non-inflammatory skin lesions as well as GAGS scores in patients with mild-to-moderate acne. Our results suggest that the clinical effects of bacteriocins from B. subtilis may be related to the capacity to promote S. aureus decolonization in acne areas. Notably, the treatment approach was safe and was associated with good satisfaction levels.
After 60 days of topically applied bacteriocins from B. subtilis, we found a statistically significant 38% decrease in the absolute abundance of S. aureus in the acneic skin. An important strength of our microbiological study is that we focused on the absolute bacterial abundance as the main outcome. Growing evidence suggests that the absolute abundance is not only paramount for species competition within microbial communities but can also be a key regulator of S. aureus virulence [19]. In this scenario, investigating the modifications in absolute rather than relative abundance is likely to have major clinical implications. In an era of increasing emphasis on the risks of resistance to antibiotic treatment [20], the microbiological activity described in our study makes the topical application of bacteriocins from B. subtilis an excellent option for rapid decolonization of acneic skin from S. aureus.
We thus speculate that the observed clinical effects of topically applied bacteriocins on the number of inflammatory and non-inflammatory lesions as well as on GAGS scores may be mediated by a reduction in the absolute abundance of S. aureus in acne areas. Although our data require confirmation in large placebo-controlled trials, our proof-of-concept evidence suggests that bacteriocins from B. subtilis may serve as a topical strategy to improve the clinical appearance and the severity of acne, potentially representing an alternative to avoid the harmful effects of systemic antibiotic therapy on the microbiota of other body sites.
The main limitations of our study include the exclusive focus on Caucasian patients, the lack of a placebo arm, and the fact that the microbiological study was performed only in a subset of participants (n = 12). Currently, our results cannot be considered as a basis for treatment recommendations. Patients with mild-to-moderate acne should be treated on an individual basis according to each patient’s characteristics based on the results of well-designed clinical trials. Our study did not compare the effects of topically applied bacteriocins with those of topical antibiotics. Finally, we specifically focused on S. aureus as the main bacterial species of interest. It is plausible that a decrease in S. aureus abundance could lead to changes in the skin bacterial community (including C. acnes), which could in turn reduce local cutaneous inflammation, although this possibility needs to be proven by further investigations.
Conclusions
The results of our microbiological and clinical studies indicate that topically applied bacteriocins from B. subtilis can promote S. aureus decolonization in acneic skin, ultimately improving the clinical appearance of mild-to-moderate acne. Further studies are necessary to determine the optimal dose and treatment duration with topical bacteriocins in acne. Future research directions include examining how the whole skin microbiota of acneic patients would change in response to topical application of bacteriocins, and determining if bacteriocins extracted from bacteria other than B. subtilis (e.g. the probiotic strain Lactobacillus salivarius LS03 [21]) might be useful for clinical use in acneic patients.








